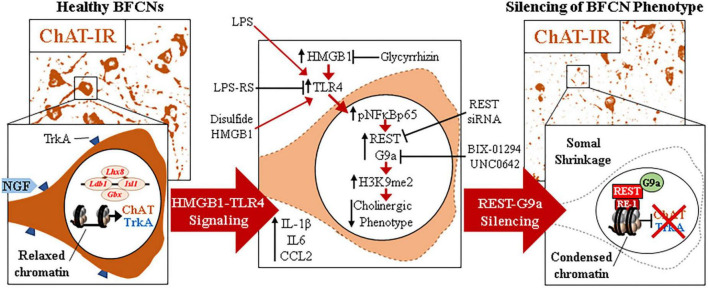
FIGURE 6.
Schematic depicting the proposed neuroimmune-epigenetic mechanism underlying epigenetic repression of the cholinergic neuron phenotype. (Left) In naïve basal forebrain, healthy basal forebrain cholinergic neurons (BFCNs) express the ACh-synthesizing enzyme ChAT, the high-affinity NGF receptor tropomyosin receptor kinase A (TrkA), the LIM/homeobox protein 8 (Lhx8), and other cholinergic phenotype and lineage genes. (Top) Photomicrographs of naïve ChAT + IR neurons in the basal forebrain slice culture (FSC) model. (Bottom) Schematic depicting a ChAT + IR BFCN in orange. The Chat and Lhx8 gene promoters contain the consensus 21-base-pair DNA binding sequence RE1 (Shimojo and Hersh, 2004; Abrajano et al., 2009). Note that the transcription factor RE1 silencing transcription factor (REST) is not bound to the RE1 binding site allowing relaxed chromatin and active transcription of Chat, Trka, Lhx8, and other cholinergic genes in healthy BFCNs. (Middle) Signaling schematic: Toll-like receptor 4 (TLR4) activation with LPS increases nuclear translocation of pNFκB p65 inducing REST, CDYL, and G9a expression as well as increased histone 3 lysine 9 dimethylation (H3K9me2) and REST occupancy at promoter regions on Chat, Trka, and Lhx8 cholinergic genes. Inhibition of HMGB1 with glycyrrhizin, TLR4 with LPS-RS, and REST knockdown with siRNA all block LPS-induced loss of ChAT. Inhibition of the methyltransferase G9a with BIX-01294 and UNC0642 prevented and restored, respectively, the LPS-induced loss of ChAT + IR BFCNs. (Right) Neuroimmune induction causes epigenetic repression of the cholinergic phenotype. (Top) Photomicrographs of LPS-treated BFCNs depicting loss of ChAT + IR neurons and somal shrinkage of the remaining ChAT+ cholinergic neurons in the FSC model. (Bottom) Schematic depicting REST binding to RE1 sites on Chat and Lhx8 genes, recruiting G9a to repress expression of Chat, Trka, and Lhx8 in BFCNs. This condenses chromatin at cholinergic genes, repressing gene transcription through a reversible mechanism. Note that expression of the neuron-specific marker NeuN and the cell death marker propidium iodide was unchanged by LPS treatment, consistent with loss of the BFCN phenotype and not cell death. HMGB1-TLR4 signaling-induced cholinergic gene repression may represent a neuroprotective mechanism resulting in the reversible loss of highly differentiated BFCNs.