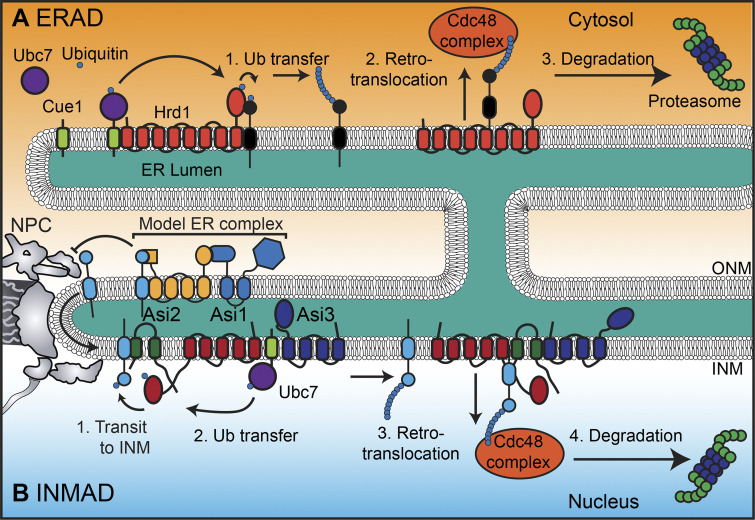
Figure 2.
ERAD and INMAD. (A) Schematic of ERAD of a hypothetical substrate. (1) Upon binding the substrate, Hrd1 receives multiple ubiquitin moieties (sequentially) from an E2 such as Ubc7 and transfers them to the substrate (black). (2) The substrate is retrotranslocated from the ER membrane into the cytosol in a manner dependent on the Cdc48/p97 complex and a retrotranslocon channel formed by Hrd1. (3) The substrate is recruited to the proteasome, where it is degraded. (B) INMAD is required for clearing INM proteins through a mechanism similar to ERAD, but instead of Hrd1, the Asi complex or other E3 ubiquitin ligase/retrotranslocon channels are used. INMAD is also used to degrade orphan subunits (light blue) of ER complexes as depicted: (1) Multiprotein complexes are too large to diffuse past the peripheral channels between the NPC and the POM, but orphan subunits can. (2) Asi2 then binds the orphaned substrate via its transmembrane domain; Asi1 receives a ubiquitin moiety from an E2 such as Ubc7 and transfers it to the substrate. (3) The orphaned substrate is retrotranslocated from the INM into the nucleus in a manner dependent on the Cdc48/p97 complex and a retrotranslocon channel likely formed by the Asi complex. (4) The orphaned substrate is recruited to the proteasome, where it is degraded.