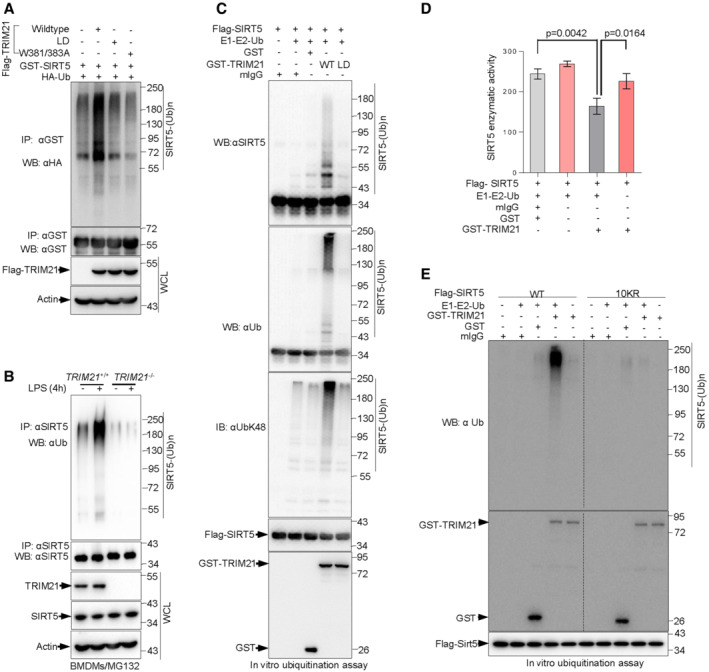
Figure 2. TRIM21 is an E3 ligase of SIRT5 promoting its ubiquitination, which is enhanced by LPS challenge.
-
AImmunoblot analysis of WCLs and anti‐GST immunoprecipitates derived from 293T cells transfected with the indicated plasmids for 48 h, cells were then treated with the proteasome inhibitor MG132 for another 4 h prior to harvesting.
-
BWCLs from TRIM21 +/+ and TRIM21 −/− BMDMs treated with 100 ng/ml LPS for 4 h were immunoprecipitated with anti‐SIRT5 antibody and analyzed by Western blotting. 10 μM MG132 was added for 4 h before harvesting.
-
CTRIM21 ubiquitinates SIRT5 in vitro. Immunopurified SIRT5 were incubated with purified recombinant TRIM21 or mutant TRIM21 proteins, E1 and E2 (UBE2D2), and ubiquitin as indicated in 30°C for 90 min. The ubiquitination reaction products were resolved by SDS‐PAGE and probed with the indicated antibodies.
-
DPurified SIRT5 protein were incubated with purified recombinant TRIM21, E1 and E2 (UBE2D2), and ubiquitin in the presence or absence of control mIgG or GST proteins as indicated for 90 min at 30°C. SIRT5 enzymatic activity was measured. Data are from n = 3 biological replicates. Data are the mean ± SD. Statistical significance was determined by unpaired two‐tailed Student's t‐tests.
-
EPurified SIRT5 and mutant SIRT5‐10KR proteins were incubated with purified E1 and E2 (UBE2D2), ubiquitin and/or purified recombinant TRIM21 or control mIgG or GST proteins as indicated at 30°C for 90 min. SIRT5 ubiquitination and TRIM21 protein levels were analyzed by Western blotting with the indicated antibodies.
Data information: All IP and WB data in this work otherwise indicated are representative of at least three independent experiments.
