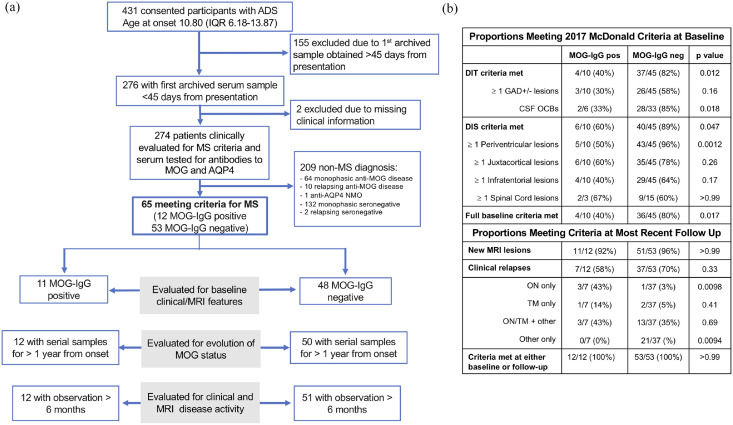
Figure 1.
Cohort selection. (a) Flow chart of study design and patient disposition. (b) Proportions of patients meeting 2017 McDonald criteria at baseline and in follow-up. Baseline criteria were evaluated on the baseline MRI (acquired within 45 days from clinical onset), in participants with non-ADEM presentation whose MRI scans were performed with administration of gadolinium-based contrast agent. Fulfillment of the McDonald criteria over time was assessed in all participants including at their most recent follow-up; median (IQR) of 8.14 (6.19–9.80) years in MOG-IgG positive participants and of 6.11 (4.06–9.25) years in MOG-IgG negative participants.
ADS: acquired demyelinating syndrome; ADEM: acute disseminated encephalomyelitis; AQP-4: aquaporin-4; CSF: cerebrospinal fluid; GAD: gadolinium-based contrast agent enhancement; MOG: myelin oligodendrocyte glycoprotein; MS: multiple sclerosis; NMO: neuromyelitis optica; OCB: oligoclonal band; ON: optic neuritis; TM: transverse myelitis.