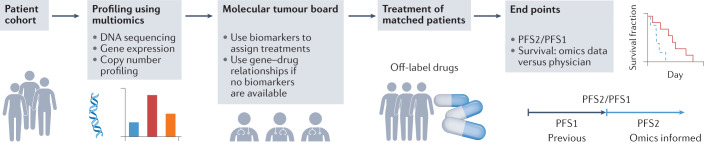
Fig. 2. Prospective clinical studies guided by omics data to use off-label drugs.
Recent umbrella clinical trials88–92 have focused on multi-omics profiling of the tumours of enrolled patients by generating and analysing genome-wide data — including data from DNA sequencing, gene expression profiling, and copy number profiling — to prioritize treatments. After multi-omics profiling, a multidisciplinary molecular tumour board led by clinicians selects the best therapies on the basis of the current known relationships between drugs, genes and tumour vulnerabilities. For each therapy, the relevant altered vulnerabilities could include direct drug targets, genes in the same pathway, indirect drug targets upregulated or downregulated by drug treatment, or other genes interacting with the drug targets through physical or genetic interactions. This process then results in patients being treated with off-label targeted therapies. The end points for evaluating clinical efficacy include the ratio of the progression-free survival (PFS) associated with omics data-guided therapies (PFS2) and the PFS associated with previous therapy (PFS1), or differences in survival between patients treated with omics data-guided therapies and patients treated with therapies guided by physician’s choice alone.