Fig. 12.
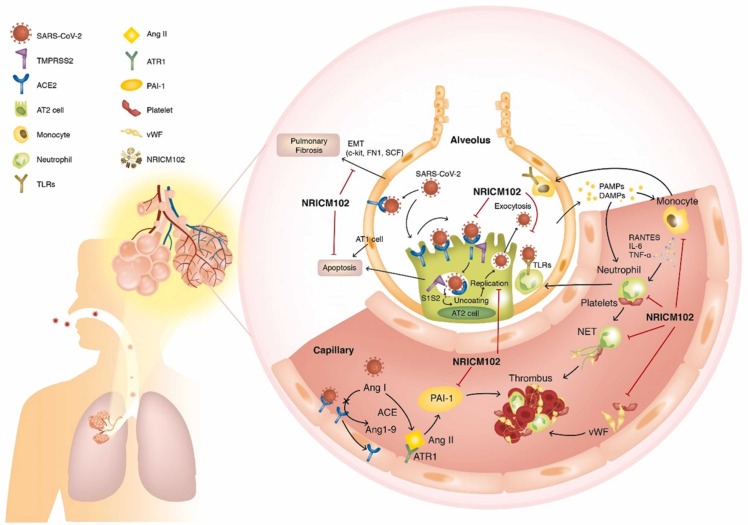
SARS-CoV-2 (S1) infection-induced alveolar type II (AT2) progenitor cell dysfunction and loss (apoptosis or necrosis) are deleterious to injured lungs for the following reasons: 1) decreases in surfactant lipids increase alveolar collapse and atelectasis; 2) decreases in AT2 cells cause impaired AT1 cell replacement, affecting alveolar repair and promoting fibrosis (EMT); 3) ACE2 downregulation by S1 drives overactivity of the ACE/Angiotensin II (Ang II)/AT1 receptor (ATR1) axis, exacerbating the tissue-destroying effects of the inflammatory responses and increasing production of plasminogen activator inhibitor − 1 (PAI-1), reducing plasmin activation and fibrinolysis; and 4) SARS-CoV-2 (S1) infection triggers AT1/AT2 and endothelial cells to release cytokines, which cause increased capillary permeability, enabling adhesion and extravasation of neutrophils and monocytes into the alveolar interstitial space. When stimulated by pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), neutrophils and macrophages secrete massive amounts of cytokines, procoagulants, and complement, which induces further vascular injury, enhancing the risk for thrombosis. Therefore, several key factors contribute to the promotion of intravascular thrombus formation. 1) Neutrophil-mediated secretion of NETs (neutrophil extracellular traps) and vWF upregulation on cytokine-/virus-activated endothelial cells or macrophages, and lung residential megakaryocytes produce locally available platelets, which enhances platelet aggregation. 2) Cytokine-triggered secretion of tissue factor (TF) by endothelial cells and macrophages stimulates the coagulation cascade and increases fibrin clot formation. 3) Overactivation of the ACE (angiotensin-converting enzyme)/Ang II (angiotensin II)/AT1 receptor (ATR1) axis increases production of plasminogen activator inhibitor-1 (PAI-1), reducing plasmin activation and fibrinolysis. Here, NRICM102 displays multitargeted effects by preventing SARS-CoV-2 S1 entry; reducing AT1/AT2 cell apoptosis; downregulating inflammatory responses involving activation of neutrophils and monocytes, release of cytokines (IL-6, MCP-1) and expression of inflammatory receptors (TLR4); and reducing the production of prothrombotic factors (vWF and PAI-1) and fibrotic factors (c-Kit, FN1 and SCF) to ameliorate pulmonary embolism and fibrosis in hACE2 mice.