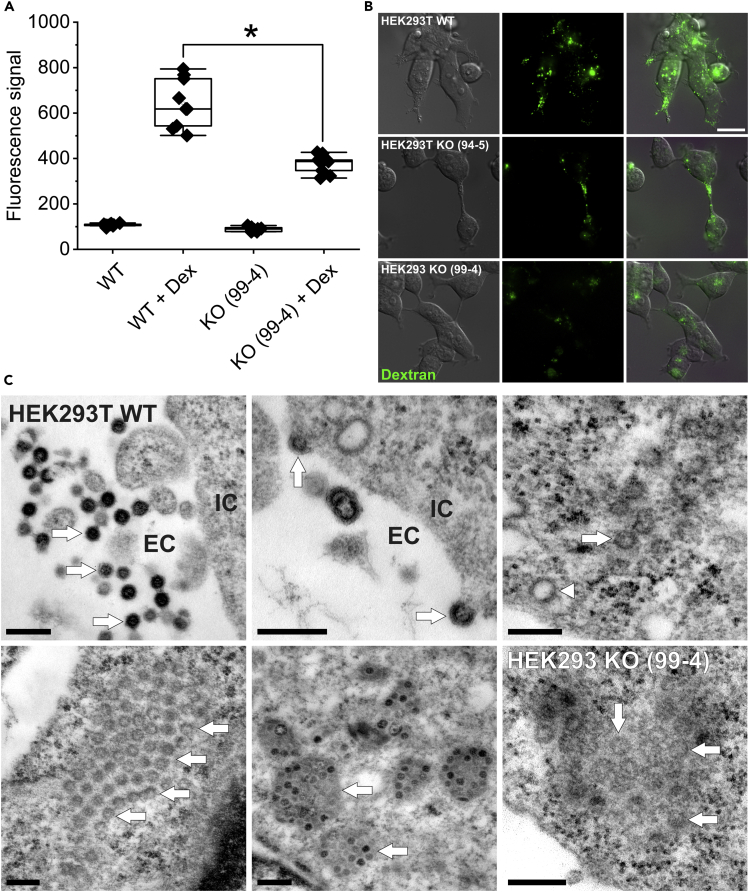
Figure 3.
Measurement of macropinocytosis and electron microscopic studies
(A) Fluorescence-coupled dextran (10,000 kDa) was used as a marker for macropinocytosis. Quantification was performed by flow cytometry. CLN7 knockout cells (KO (99-4) + Dex) exhibited a 41% reduced macropinocytosis rate compared with wild-type cells (WT + Dex). In contrast, the autofluorescence of the cells at 647 nm was the same (WT and KO (99-4)). Macropinocytosis is discussed as a mechanism for uptake of SARS-CoV-2 into cells. ∗ Significant compared to wild-type cells.
(B) Comparing fluorescence images (top row: WT, middle row: KO 94-5, bottom row: KO99-4), a reduced number of macropinosomes (green) can be observed in both knockout cell lines. Scale bar: 20 μm.
(C) Electron microscopic studies. Top left: SARS-CoV-2 particles are in the process of infecting a HEK293T cell. The viruses (three exemplary arrows) are located in the extracellular space (EC). The cell is cropped in the right part of the image (IC = intracellular space). As described in literature, the particles are between 80 and 120 nm in size. Top center: viruses which are about to enter the cell (arrows). Top right: Intracellularly, the viruses always appear in groups (arrow points to a virus particle as an example). They can be easily confused with clathrin-coated vesicles (arrowhead), which have a similar size. Bottom left: A group of viral particles in the cytosol (arrows). The ERGIC membrane envelope is not visible with this method of contrast. Bottom center: In some images, the contents of ERGIC vesicles as well as the viruses themselves are clearly more electron dense (two exemplary arrows), possibly indicating a different stage of maturation. Bottom right: viruses were also found in CLN7-deficient cells, but at a much lower abundance. Scale bar: 250 nm.