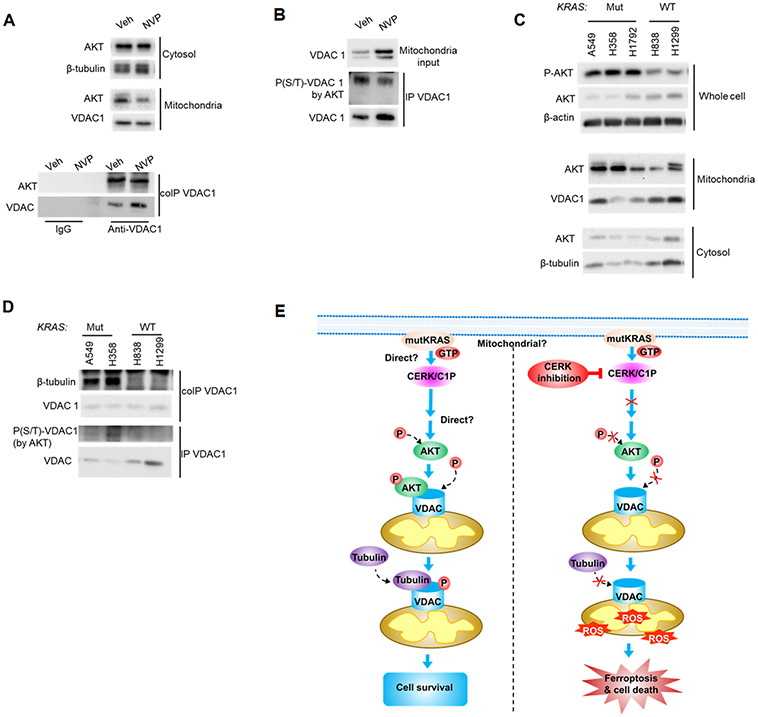
Figure 8: Ceramide kinase inhibition decreased mitochondrial AKT level, AKT-VDAC binding and AKT-mediated VDAC phosphorylation.
(A, B) A549 cells (LUAD-derived) were treated with vehicle (Veh) or NVP 231 (400 nM) for 24 hours, mitochondria fraction prepared from the post-treatment cells were subjected to co-IP (A) or IP (B) assay for VDAC1 followed by SDS-PAGE/immunoblotting; cytosol and mitochondria fraction was also included in the Western immunoblotting. (C, D) Compared to WT KRAS NSCLC cells, Mut KRAS cells had more AKT activation, less VDAC-tubulin binding and AKT-mediated phosphorylation level of VDAC1. A549/H358/H1792/H838/H1299 cells at approximately 40-50% confluency were placed in serum-free media for 16 hours, then cell lysates were prepared and utilized in SDS-PAGE/immunoblotting (C) or mitochondria fractions prepared from the cell samples were subjected to co-IP or IP assay for VDAC1 followed by SDS-PAGE/immunoblotting (D). (E) Mechanistic model of how CERK inhibition attenuates Mut KRAS NSCLC cell survival. Left panel: in NSCLC cells with constitutively active KRAS by mutation, high CERK activity/C1P level elevates PI3K signaling and activates AKT by phosphorylation; active AKT then transports to mitochondria where it interacts with and phosphorylates VDAC; phosphorylation of VDAC by AKT results in more tubulin recruitment to VDAC, which reduces VDAC function and ROS generation to limit ROS-induced ferroptotic cell death. Right panel: low level or the absence of CERK/C1P prevents AKT activation, VDAC phosphorylation by AKT and subsequent VDAC-tubulin interaction; with less tubulin binding, VDAC activity were enhanced, causing more ROS production, ROS-mediated ferroptosis and cell death.