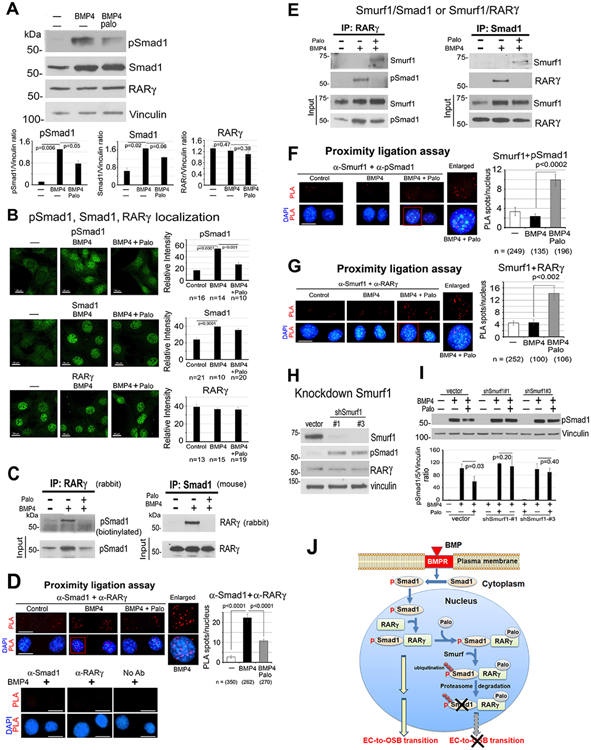
Figure 3. Palovarotene decreases BMP4-stimulated pSmad1.
(A) pSmad1, Smad1 and RARγ protein levels in 2H11 cells treated as indicated for 6 h. Vinculin, loading control. Blots quantified by ImageJ. (B) Immunofluorescence of pSmad1, Smad1, and RARγ in nucleus of cells in (A). Relative intensity quantified by ImageJ. n, number of nuclei examined. All bars, 20 μm. (C) Co-immunoprecipitation of RARγ and pSmad1 from nuclear extracts. (D) Proximity ligation assay for the interaction of RARγ and pSmad1. Red spots, PLA signals in the nucleus using anti-Smad1 and anti-RARγ antibodies. Single or no antibodies were used as controls. (E) Co-immunoprecipitations of Smurf1 with RARγ or pSmad1 using nuclear extracts. Note in (C, E), efficiency of immunoprecipitations could not be assessed due to RARγ and Smad1 being similar in size as IgG bands. (F-G) PLA for Smurf1 with pSmad1 (F) or RARγ (G). (H) Smurf1 knockdown in 2H11-shSmurf1#1 or #3 clones. (I) pSmad1 levels in 2H11-shSmurf1 clones. (J) Graphical summary. BMP4 stimulates an increase in pSmad1 that translocates into the nucleus, where pSmad1 and RARγ form a complex. Palo results in the recruitment of E3-ubiquitin ligase Smurf1 to the pSmad1/RARγ complex, leading to pSmad1 degradation and inhibition of EC-to-OSB transition.