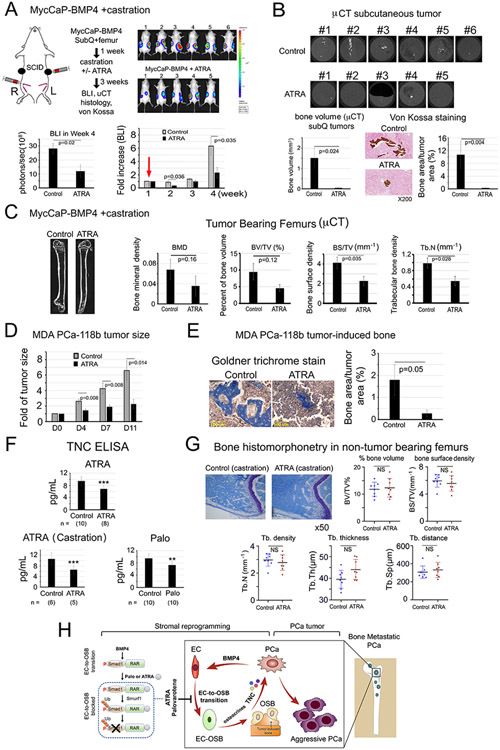
Figure 6. ATRA reduces PCa-induced bone formation and tumor growth of MycCaP-BMP4 and MDA PCa-118b tumors, reduces plasma TNC levels, but does not lead to overall bone loss in non-tumor bearing femur in castrated mice.
(A) MycCaP-BMP4 cells (0.25 x106/site) were implanted subcutaneously and intrafemorally. Mice were castrated 1-week post-implantation (red arrow) and treated 3-weeks with or without ATRA. Tumor size measured by BLI. Control (n=6); ATRA-treated (n=5). (B) μCT of ectopic bone in tumors grown subcutaneously. Mineralization by von Kossa staining. (C) μCT of tumor-bearing femurs from mice in (A). (D) Tumor size of MDA PCa-118b with or without ATRA. Control (n=7); ATRA-treated (n=7). (E) Mineralization by Goldner Trichrome staining. (F) Serum TNC protein levels from SCID mice treated with ATRA, ATRA+castration, or Palo were quantified by ELISA. n, number of mice analyzed. (G) Bone histomorphometry on non-tumor containing femurs from ATRA plus castration-treated mice in (A). (H) Model. Within the tumor microenvironment, PCa-induced bone originates from ECs that have undergone EC-to-OSB transition in response to PCa-secreted BMP4 signaling through a pSmad1/RAR pathway (boxed). Activation of RARs by Palo or ATRA inhibits BMP4-mediated EC-to-OSB transition through a non-canonical RAR/pSmad1/Smurf1 pathway that results in pSmad1 degradation (dotted). This Palo/ATRA-mediated inhibitory pathway blocks stromal reprogramming and leads to a decrease in EC-OSB cell secreted factors (“osteocrines”) including TNC, a reduction in PCa-induced aberrant bone formation, and a decrease in metastatic PCa tumor growth in bone.