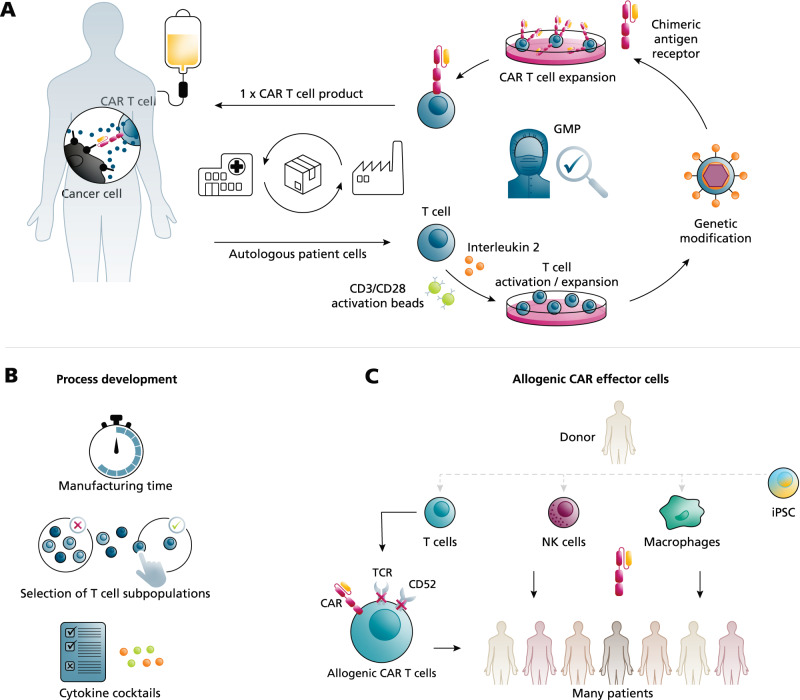
Fig. 1. Manufacturing of CAR T cells and biological approaches to increase the availability of CAR immunotherapies.
A Currently approved autologous chimeric antigen receptor (CAR) T cell immunotherapy is based on a person’s own T cells that are given back to the same patient after ex vivo modification. The production process is performed under Good Manufacturing Practice (GMP) conditions and monitored by several quality controls. B Biological approaches to increase CAR T cell quality by process development include the reduction of the manufacturing time, the selection of efficient T cell or CAR T cell subpopulations and the required cytokine combinations in order to avoid exhausted cells. C For allogenic CAR immunotherapy, different immune effector cells are obtained from healthy donors and can be given to a large cohort of patients. To enable allogenic CAR T cells, depletion of their T cell receptor (TCR) and the CD52 molecule is performed before they are further modified with the CAR. Other promising immune effector cells are natural killer (NK) cells or macrophages. To obtain cells for allogenic CAR cells, mature healthy donor cells could be derived from induced pluripotent stem cells (iPSC) in the future.