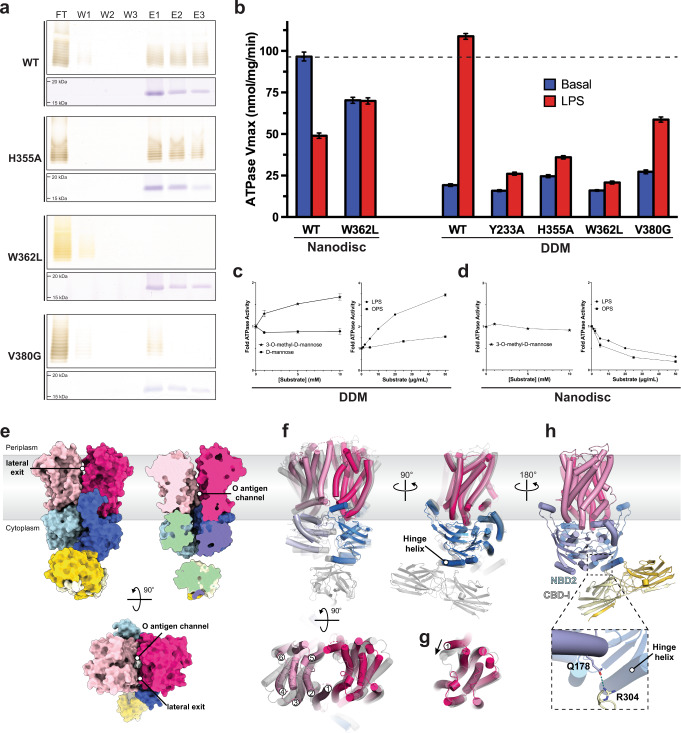
Fig. 6. Site-directed mutagenesis disrupts LPS binding and O antigen modulated ATPase activity.
a SDS-PAGE of pulldown experiments examining A. aeolicus LPS binding against different CBD mutant constructs. Top and bottom panels are silver and Coomassie-blue stained gels, respectively. Uncropped images are provided as Source Data. b ATP hydrolytic activity of WzmWzt and O antigen binding mutants determined in nanodiscs and DDM micelle. WzmWzt ATPase activity measuring the maximum velocity among DDM-solubilized transporter and nanodisc reconstituted constructs in the presence (LPS) or absence (Basal) of saturating A. aeolicus LPS. Data presents the maximum velocity measured from Michaelis Menten non-linear fit model. Each data point is the means from 3 technical repeats. Error bars represent the upper and lower 95% confidence limits from the Michaelis-Menten non-linear fit (profile likelihood) c WzmWzt ATPase activity in a DDM micelle titrating 3-O-methyl-mannose, D-mannose, LPS, and O antigen. Data points represent averaged activities from three replicas normalized to the activity in the absence of substrate. Error bars represent the deviations from the means. d As in panel C but using nanodisc reconstituted WzmWzt. Individual data points are provided as Source Data. e Structure of WzmWzt in a DDM micelle shown as a surface colored according to the individual domains. Data points and error bars as stated under panel (c) f Superimposition of channel-forming nucleotide-free conformations of WzmWzt. The full-length transporter is shown in gray, the CBD-truncated construct (PDB: 6OIH) is colored according to its domains. g TM helix 1 adopts different conformations in CBD-truncated (magenta) and full-length (gray) apo WzmWzt. The structures were aligned on one Wzm subunit and the other domains are not shown for clarity. h Interactions between NBD2 and CBD2-i in the in surfo full-length WzmWzt structure.