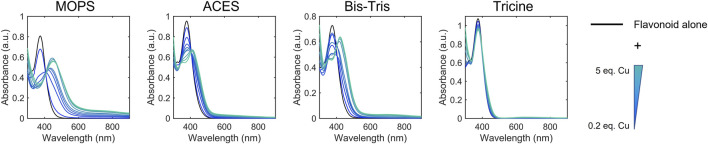
FIGURE 3.
Representative electronic absorbance spectra of QT and CuSO4 in various 50 mM buffers (pH 7.4) for determining binding affinity ranges. CuSO4 was titrated into a 50 μM solution of QT from 0.2 to 5 equivalences. Spectral changes correspond to binding between QT and Cu(II) which indicates that QT has a stronger binding affinity than the corresponding buffer. Conversely, the lack of spectral changes upon Cu(II) addition in tricine buffer indicates that the binding affinity between tricine and Cu(II) is stronger than that of QT and Cu(II). These experiments allow us to estimate the binding affinity of QT and Cu(II) to be between that of BisTris-Cu(II) (5.3 μM) and tricine-Cu(II) (50 nM). Electronic absorption spectra of the remaining flavonoids tested are shown in Supplementary Figure S1.