Abstract
Gold nanoparticles are synthesized from alpinia calcarata extract. The synthesized nanoparticles are considered for their structural, morphological and nonlinear optical properties. Powder X-ray diffraction analysis revealed the structural purity of the prepared samples. FTIR confirmed the presence of biomolecules involved in the reduction and stabilization process. UV–visible spectroscopic studies confirmed the Surface Plasmon Resonance of the prepared nanoparticle. HRTEM exposed the spherical shape morphology of the prepared gold nanoparticles. Zeta potential analysis inferred the stabilization of gold nanoparticles. The synthesised gold nanoparticles are found to be poly-dispersed with an average size of 15 nm. The studies suggest that the glucose and its complex in the alpinia calcarata extract are responsible for the reduction nanoparticles, whereas proteins act as capping agents around the nanoparticles. The Z-scan studies discovered the nonlinear optical behaviour and thus measured its parameters.
Keywords: Gold nanoparticles, Nonlinear optics, Green synthesis, Z-scan technique
Gold nanoparticles; Nonlinear optics; Green synthesis; Z-scan technique.
1. Introduction
In current scenario, green routed nanoparticles has garnered wide interest owing to its intrinsic features such as eco-friendliness, cost effective and easy synthesis method. Synthesis of nanoparticles by chemical routes possesses challenges due to the fact that colloidal solutions are contaminated with several by-products of chemical reactions [1]. Considerable efforts have been made to develop environment friendly protocols for the nanoparticles synthesis [2]. The tremendously amazing functionalities of gold nanoparticles (NPs) have massive potential on present technology and accentuating their uses in human welfare. Presence of surface plasmon resonance (SPR) in the gold NPs have significant applications in many fields [3, 4]. Gold NPs exhibit significant bio activities and catalytic support [5, 6, 7], photonic [8] and SERS [9, 10] applications. It is well known that conventional methods of synthesis of gold NPs are usually slow and involve use of toxic reducing agents such as trisodium citrate, sodium borohydride and dimethyl formamide, which pose environmental burden [11, 12, 13, 14]. Hence, the green methodology is widely used to overcome above said confines [15, 16, 17, 18, 19]. Alpinia calcarata is as an effective medicinal plant with strong antibacterial, antifungal, and antinociceptive activities [20]. Alpinia calcarata is one of the potential source for the production of metal nanoparticles [21, 22].
The unique nonlinear optical properties of metal nanoparticles are widely used in nonlinear spectroscopy, fibre optic communications and quantum memories [23]. Third order nonlinear property has been studied on gold NPs prepared by chemical synthesis method [24, 25]. Morphology dependant non-linear optical properties have been reported for Au NPs [26, 27, 28]. Recently, high-order harmonic generation in Au NPs has been studied [29]. Gold NPs conjugated with methylene blue showed improved third order non-linear properties [30]. However, green synthesised metal nanoparticles possess promising non-linear optical properties [31]. Nonlinear behaviour were checked for different materials were performed using solutions and thick films. Meanwhile it is fascinating to examine in nanoparticles (of order of a hundred nanometers) [28]. Third order optical nonlinearity has already been reported on silver nanoparticles in our previous study [31, 32]. In this paper, we have synthesised green approached gold nanoparticles using Alpinia calcarata root extract and prepared gold nanoparticle shows that good stability even at room temperature, the size and shape of the nanoparticle can be controlled by varying the volume and concentration of the extract. As synthesised gold nanoparticle shows nonlinear optical behaviour hence, it is demonstrated as optical limiting application aspects.
2. Materials and methods
2.1. Materials
The pure form commercial grade Gold (III) chloride trihydrate solution (HAuCl4 3H2O) was procured from Sigma–Aldrich chemical company. Alpinia calcarata root was acquired from local Ayurvedic store, India. In the current study the double distilled water was used for synthesis process.
2.2. Methods
The purchased A. calcarata roots were washed thrice and dried in sunshade for three days and grinded as fine powder. 10 gm milled powder was taken in a beaker having 200 ml of D. Distilled water, the mixture were stirred continuously at 70 °C for 30 min then the mixture were filtered using filter paper (Whatman No.1). To synthesis AuNPs nanoparticles the filtrate (A.calcarata extract) was used.
2.3. Gold nanoparticles synthesis
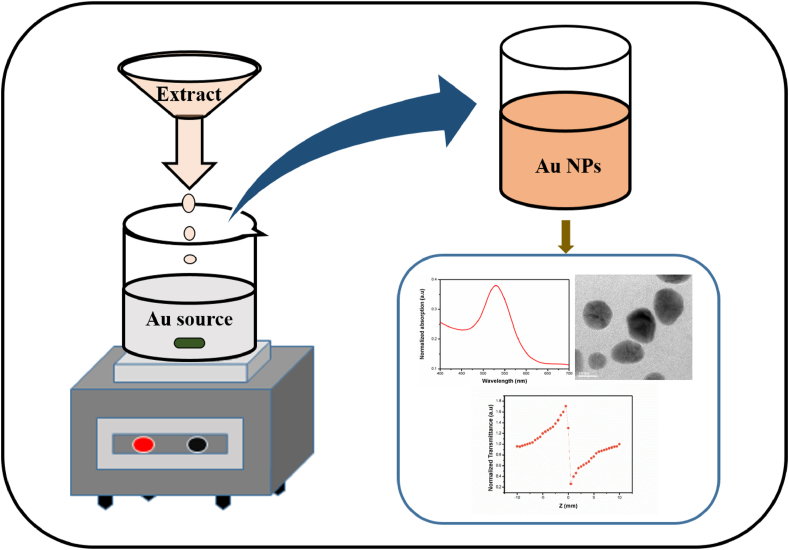
The Gold (III) chloride trihydrate (HAuCl4 · 3H2O) is used as precursor to prepare gold nanoparticles at the room temperature using resultant filtrate extract. In order to understand the quantity of the extract role in the synthesis process, different quantity of extract were used in the process of nanoparticle preparation. 0.2 mM concentration of gold source was used in the entire experiments. Figure 1 shows the schematic sketch of the synthesis process. In every 100 ml of gold source was used for various volume of extract like 0.5, 0.75, 1 ml of A. calcarata root extract. The drop wise addition was followed for all experiments. The addition rate of the extract was maintained for all experiments was 0.025 ml/minutes and then the stirring was continued for 1 h after the completion of addition of extract. The dispersity of nanoparticles depends on the rate of addition of extract in precursor hence, its plays an important role on the dispersity. To control the dispersity of Au nanoparticles, the addition rate of extract was varied in one experiments. The colour changes indicates the formation of gold nanoparticles. The obtained colloidal form of gold nanoparticles was used for further characterization. The pH value of the reaction solution is 7 < 9, it varies with reaction time. Since the solution is alkaline nature, the time duration for formation of nanoparticles is very less. The formation of nanoparticles ended up within 25 h at room temperature.
Figure 1.
Schematic diagram of Au NPs synthesis process.
2.4. Characterizations
As prepared colloidal gold nanoparticles were subjected into centrifugal action to separate the particle from its colloidal form and the particles were dried in air oven. The powder X-ray diffraction analysis has been done to confirm the purity and crystalline nature of the prepared gold nanoparticles. The XRD pattern for dried AuNPs was recorded using XRD (BRUKER D2 PHASER) Unit operated under the condition of 30 kV with CuK2 radiation (1.542˚A) between 2θ angle (10–80◦). The UV–Vis spectrophotometer (Shimadzu) has been used to monitor the Surface Plasmon Resonance band of the synthesized colloidal AuNPs. The High resolution transmission electron microscope (HRTEM, JEOL JEM 3010) was used to analyse the particle size and shape of the synthesized AuNPs and elemental composition has been identified in HRTEM equipped with EDX (JEOL JEM 3010). The functional groups which is present in the aqueous medium are responsible for reduction and stabilization of ions and are identified using Infra-Red spectrum, and the spectrum was recorded in Perkin Elmer Fourier transform infrared spectrometer with a resolution of 4 cm−1. The stability of synthesised gold nanoparticles were measured using ZEN 3600 for zeta potential measurements. A single beam Z-scan technique was used to analyse the nonlinear behaviour of the prepared gold nanoparticles (wavelength of 532 nm using Nd:YAG (SHG) Continuous wave laser).
3. Result and discussions
3.1. Crystalline structure
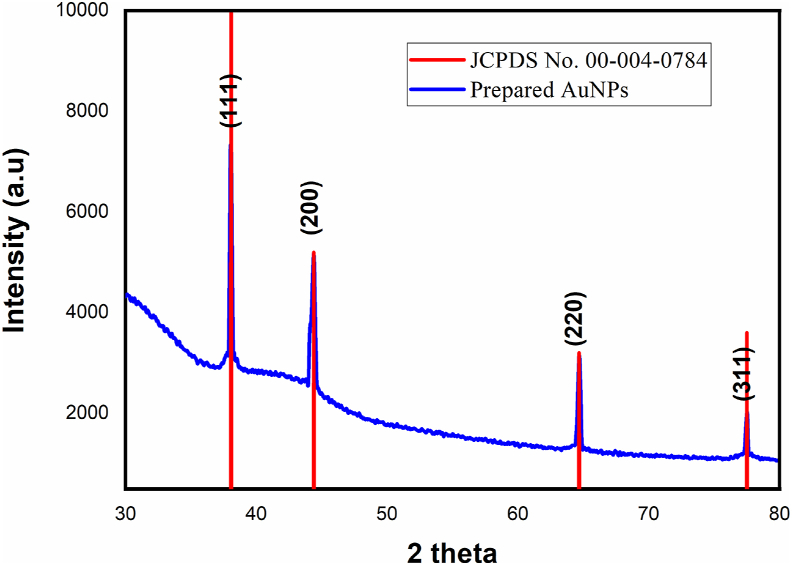
The crystalline nature of the gold nanoparticle were revealed in XRD pattern (Figure 2) peaks at angle 38.09, 44.36, 64.67 and 77.54 with indexed planes (111), (200), (220) and (311) and matched with the database of the Joint Committee on Powder Diffraction Standards, USA (JCPDS No. 00-004-0784). Scherrer's formula was used to find the average crystallite size of the prepared nanoparticles and it is found that as 21 nm. No other peaks of crystallographic impurities were found in the sample and it is confirming that the synthesized AuNPs are composed of pure crystalline gold particles of cubic face centred structure. These results are matched with previous studies of gold nanoparticles results reported [25].
Figure 2.
XRD pattern of synthesised Au NPs using A. calcarata.
3.2. Surface plasmon resonance analysis
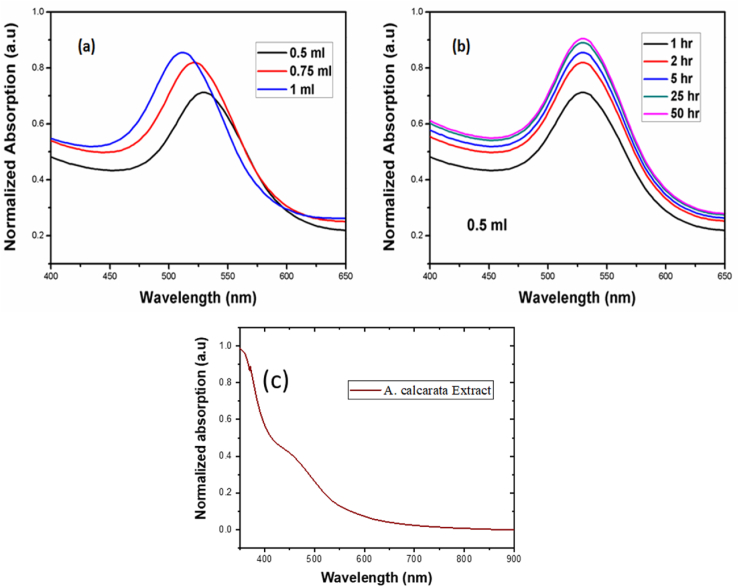
The change of refractive index of the medium was analysed by the surface plasmon resonance (SPR) which is recorded by UV- visible spectrophotometer and it is an optical method to measure formation of gold nanoparticles and how binding analyte molecules with receptor molecules on the metal surface [33]. This feats the generation of surface plasmon resonance in the surface of metal, and produces a momentary wave that extents a short distance in the colloids due to the total internal reflection of light at a metal surface-solution interface. The formation and stability of gold nanoparticles from the gold metal ions reduced by extract was monitored on a UV–visible spectrometer. The addition of extract speedily initiated the change of colour from the light yellow to advent of a red wine colour, indicating the formation of AuNPs [34]. The colour change was accrued due to excitation of surface Plasmon vibrations with formed AuNPs. The size and shape of the particles is depends on the SPR absorbance and its peaks confirmed the formation of AuNPs. The ability of alpinia calcarata extract to form nanoparticles was studied in a periodic manner. Figure 3(a) shows UV–vis spectra of the prepared nanoparticles with different volume of the extract. The volume of the extract was played in size formation of nanoparticles from this UV-visible spectrum the SPR band shifted towards the UV region. The blue shift indicates that the size of particles decreases with increasing the quantity of the extract because simultaneous reduction and capping of the nanoparticles. The surface plasmon resonance band obtained at 529, 521, 512 nm for different quantity of extract used to synthesis 0.5, 0.75, 1 ml respectively. The SPR band of the prepared gold nanoparticles were monitored with different time interval for 0.5ml extract addition. Figure 3(b) shows the SPR band of the gold nanoparticles with different time period from this spectrum growth count of nanoparticles increases with reaction time. The trace of 25 h and 50 h almost overlapped with each other, from this spectrum it can be concluded the reaction was completed within 25 h. The significant colour (ruby red) changes and characteristic of UV–vis spectra are known to be due to the phenomenon of surface plasmon resonance (SPR) displayed by AuNPs and UV-Visible spectra for alpinia calcarata extract shown in Figure 3(c).
Figure 3.
SPR Band of synthesised Au NPs Using A. calcarata, (a)-0.5,0.75,1ml of extract addition, (b)-0.5ml of extract addition for different time interval, (c)-UV spectra of A. calcarata extract.
3.3. Functional group analysis
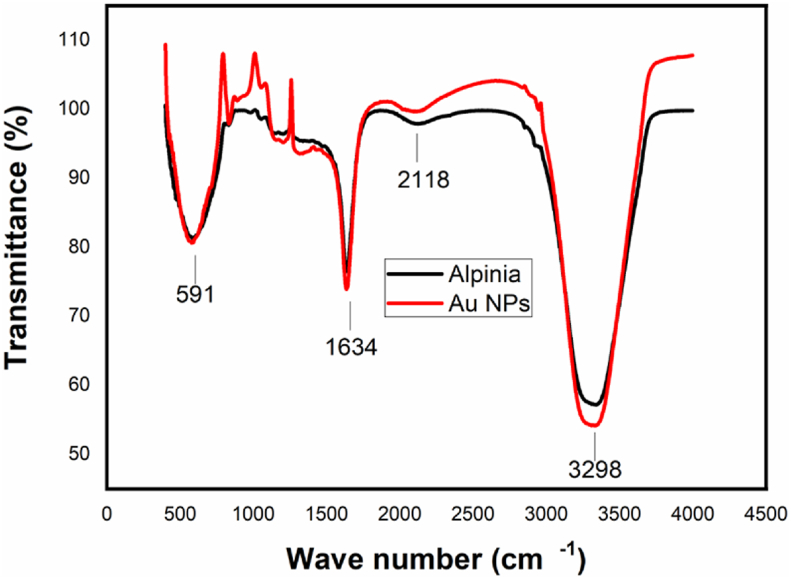
The FTIR spectroscopic analysis has been done to identify the responsible functional groups for reduction and stabilization of the prepared gold nanoparticle. The functional groups absorbs infra-red radiation with its characteristic frequencies. Infra-Red spectroscopy is an important and typical equipment to interpret the structure and identify the compounds which is involved in the process. The amide and proteins are active in the infra-red region and its gives well-known signatures [35]. The phenolic, carboxylic, nitrogen compounds are playing important role in reduction and stabilizing processes. Presence of flavonoids (C=O bonds (carboxylic acids and its derivatives) and O–H bonds (phenol, alcohol)) and other phenolics could be reason for the formation of nanoparticles by reducing its ions and proteins can bind the AuNPs through either its free amine groups or cysteine residues [36], Hence of the AuNPs become stable due to the presence of proteins at the surface in the present green route methods [37]. Flavonoids and terpenoids characteristics peaks are observed at 591, 1634, 2118 and 3298 cm−1 in IR spectrum as protuberant peaks. The presence of such a compounds in the extract leads the reduction of gold ions and stabilize the gold nanoparticles. Figure 4 shows the recorded IR spectrum. The strong absorption band at 591 cm−1, medium absorption band at 1634 cm−1 were observed due to C–H bending vibration and C=O stretching vibration of carboxylic group respectively. The broad peak located at 3298 cm−1 could be assigned to the O–H stretching vibrations, which is indicating that the presence of hydroxyl groups in the extract [38]. Comparison of extract and solution of gold nanoparticles, the absorption peaks revealed that shifts in the extract and colloidal AuNPs medium and it is anticipated that the shifts in peak position is related to adsorption of extract constituents onto the gold nanoparticles surfaces. The likeness between colloidal solutions and extract indicated that the same compounds existed in both medium. Hence, from the above FTIR spectra details, it was evident that the presence of various biomolecules in the A. calcarata extract played a most important role in the reduction and stability of AuNPs.
Figure 4.
FTIR Spectrum of A.Calcarata and AuNPS.
3.4. Surface morphological analysis
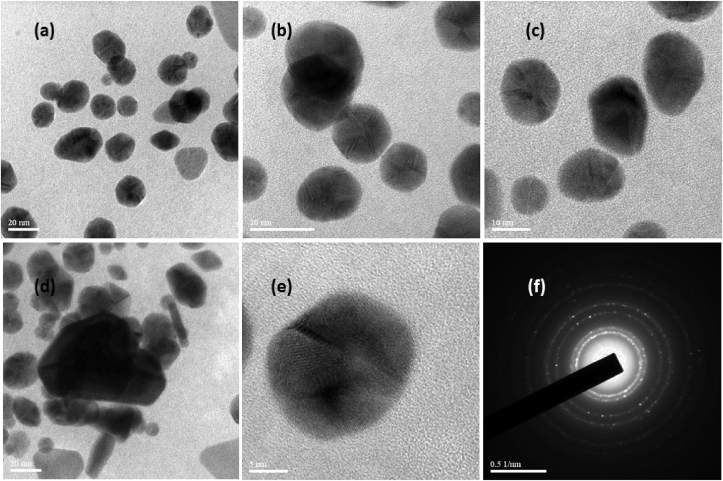
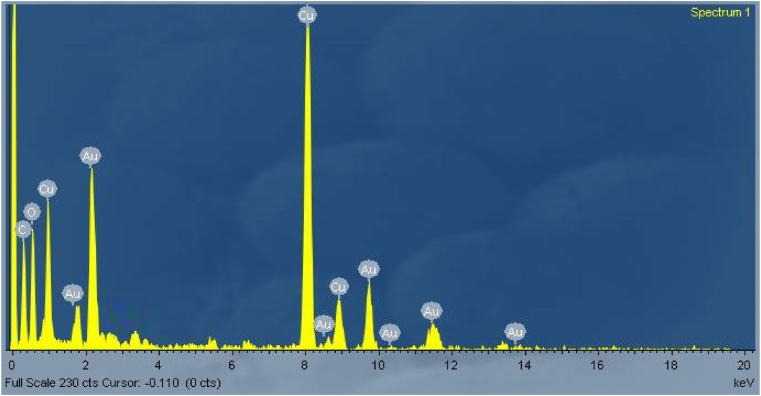
The surface morphology of the prepared AuNPs were analysed using high resolution transmission electron microscope (HRTEM). The size of Au nanoparticle varies with the quantity of A. calcarata extract. High resolution TEM images show that the synthesised AuNPs are narrow size distributed with spherical shape (varies from 20 to 25 nm). This green route methods leads to produce high stable and significant reproducible metal nanoparticle depends on the extract which was involved in synthesis process, and it is found that the quantity of extracts increases, the particle size decreases because of simultaneous reduction and capping process. The HRTEM images depicted the size and shape of the prepared gold nanoparticles with dispersity. Figure 5(a),(b),(c) shows the nanoparticles synthesised using 0.5,0.75,1 ml A. calcarata extract with uniform addition rate of extract Figure 5(d) shows the sudden addition of the extract from these images the dispersity of the nanoparticles depends on the rate of addition extract. The synthesised gold nanoparticles are crystalline (Figure 5(f)) as revealed by the SAED pattern. The diagonal lengths of the individual particles gives the maximum size of the respective particle. The presence of protein, polyphenolic and flavonoid groups in the extract prevents aggregation of AuNPs. Since the particles are spherical shape the edges were lighter rather than the centre in the HRTEM images. Intrinsic capping compounds gives the stability of the nanoparticles which is additional profit of the green synthesis method [33, 39]. The HRTEM image (Figure 5(e)) indicate the atomic structure of A. calcarata facilitated gold nanoparticle with its lattice fringes. The elemental components existing in the prepared nanoparticles were identified by Energy-Dispersive X-ray spectroscopy (EDX) armed with (HRTEM). The purity of the prepared gold nanoparticles were noticed from EDX profile (Figure 6) and the elemental percentage were tabulated in Table 1. This profile exhibits the intense peak in the gold region confirming the development of AuNPs. And other spectral signals may be due to extracellular organic moieties from extract and signal for Cu is grid which we used for analysis.
Figure 5.
HRTEM images of synthesised gold nanoparticles. ((a),(b),(c) - 0.5,0.75,1ml of extract (uniform addition), (d)-0.5ml of extract (sudden addition), (e)- lattice fringes pattern,(f)-SAED Pattern.
Figure 6.
EDX Profile for Au nanoparticles
Table 1.
Element percentages obtained from EDX.
| Element | Weight % | Atomic % |
|---|---|---|
| C K | 9.12 | 33.87 |
| O K | 9.14 | 25.48 |
| Cu K | 46.55 | 32.68 |
| Au L | 35.19 | 7.97 |
| Total | 100.00 |
3.5. Zeta potential measurements
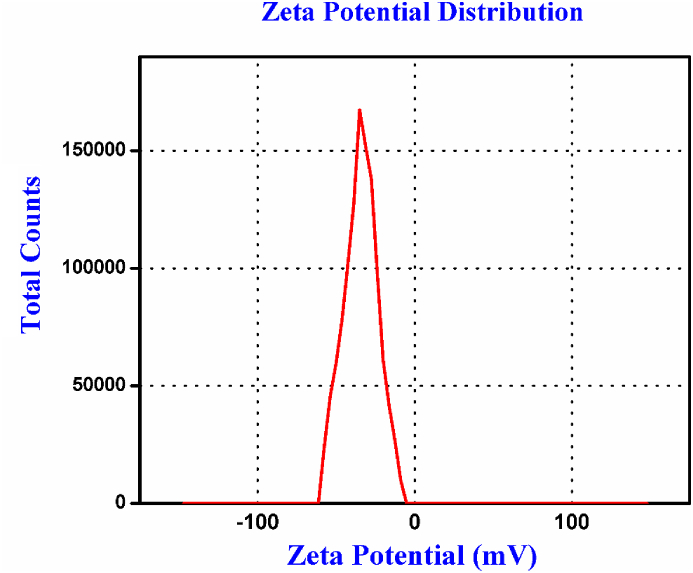
Zeta Potential analysis is used to determine the surface charge of prepared colloidal gold nanoparticles. This measurements was carried out at the angle of 90◦ with reference to the incident beam at 37 °C temperature. The zeta potential measurement is used to characterizing the outer, diffuse part of the double layer of nanoparticles. In General nanoparticles have a characteristic surface charge that can fascinate a single layer of opposite charge ions and these twofold layer ions lead with the metal nanoparticle and it diffuses in the solution and the electric potential at the surface of the twofold layer is called as Zeta potential of the prepared nanoparticles and has normally between +100 mV and -100 mV. Synthesised nanoparticles possess electric potential values in the range between +25mV and -25 mV considered as high stable nanoparticles [40]. The charge distribution contributes towards the stabilization of nanoparticles. From the Zeta potential profile (Figure 7) the measured potential value is -35 mV and hence the prepared gold nanoparticles have high degrees of stability.
Figure 7.
Zeta potential of Synthesised Au NPs nanoparticles.
3.6. Nonlinear optical studies
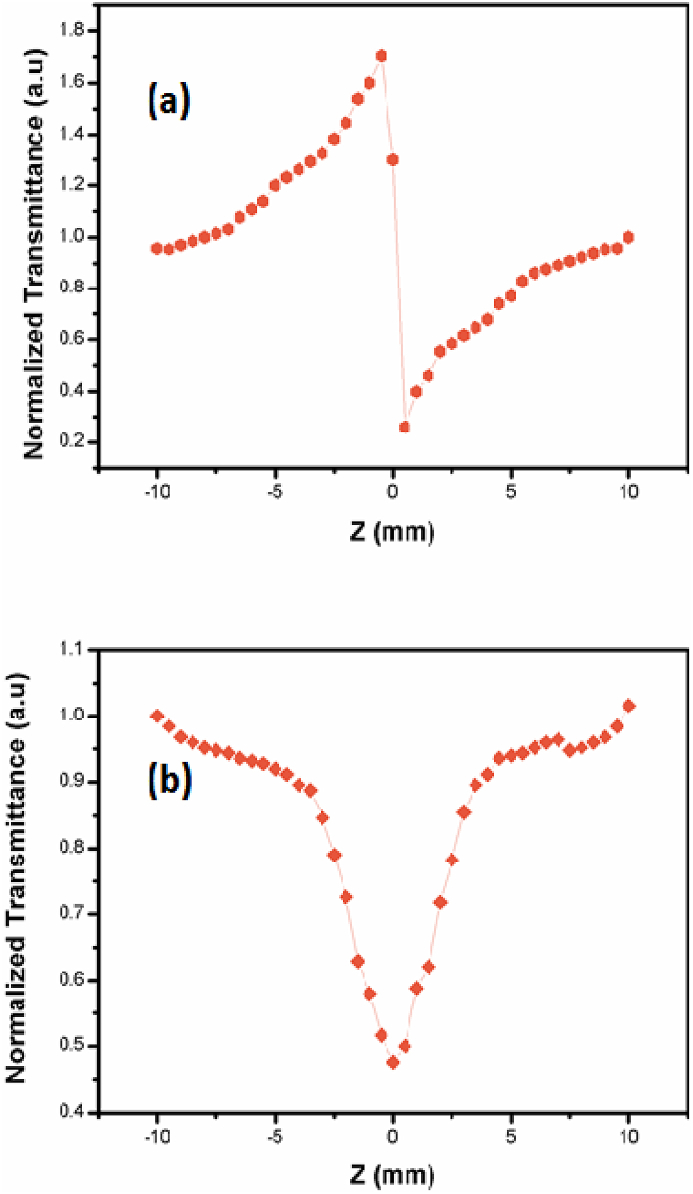
As synthesised Gold nanoparticles were analysed for nonlinear optical behaviour using Z-scan experiment. The single beam Z-scan technique gives the nonlinear refractive index and its sign of the prepared materials [41]. The Nd:YAG (CW) laser beam has focused through 3.5 cm lens. The 1 mm thick quartz cuvette containing gold nanoparticles moved in the propagation direction in the focal region (+z to −z). An intensity of the transmitted beam from gold nanoparticles were measured in closed aperture Z-scan method through an aperture. Due to the self-lensing effect irradiation of the beam with gold nanoparticles is increasing while moving towards the focal point and decreasing away from the focal point. As synthesised gold nanoparticles exhibits the negative nonlinear refractive index which is confirmed by the normalized transmittance curve characterization Figure 8a. The nonlinear refractive index n2 and nonlinear absorption coefficient β is measured from respective closed aperture and open aperture Z-scan studies (Figure 8 (b)) [42, 43, 44].
Figure 8.
Z Scan Profile of Au nanoparticles (a) closed aperture. (b) Open aperture.
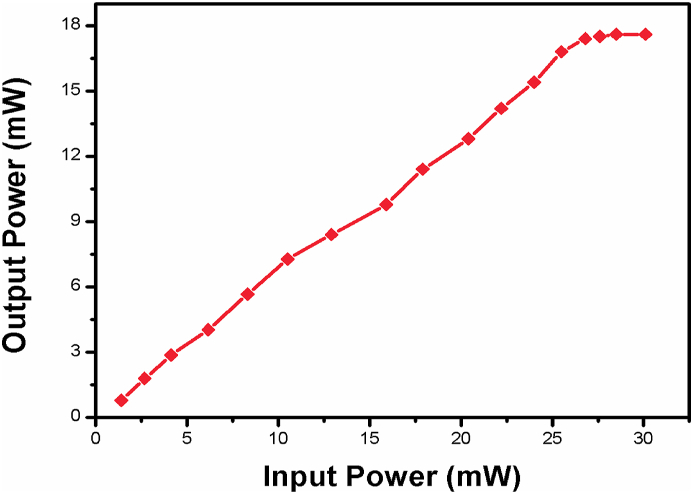
The nonlinear optical parameters of the prepared gold nanoparticles were calculated and tabulated in Table 2 and also compared with previous reported values [27] from that it is concluded as synthesised gold nanoparticle exhibit the good optical nonlinear behaviour. The output beam were collected through an aperture and its intensity is linearly with increase with increment of input intensity up to particular value then there is no variation occurs with increment in the intensity hence, the colloidal form gold nanoparticles exhibiting the optical limiting property. The negative nonlinearity of the prepared Au-NPs can be used as optical limiter. The optical limiting behaviour shown in Figure 9.
Table 2.
Measured nonlinear optical parameters of AuNPs.
| ΔTp-v | ΔΦo | n2 (×10−8 cm2/W) |
ΔT |
β (×10−3 cm/W) |
Re(χ3) [×10−4 (esu)] |
Im(χ3) [×10−4 (esu)] |
χ3 [×10−4 (esu)] |
|---|---|---|---|---|---|---|---|
| 1.443 | 3.564 | 7.122 | 0.524 | 3.353 | 3.197 | 0.636 | 3.259 |
Figure 9.
Optical limiting trace of prepared gold nanoparticles.
4. Conclusion
Gold nanoparticles (Au3+ to Au0) has been prepared using A. calcarata root extract. The aqueous extract acted as reducing as well as stabilizing agent. The addition of extract plays an important role in the dispersity of gold nanoparticles and the anticipated size can be achieved by varying the quantity of the extract. By varying the quantity of plant extract, size of the gold nanoparticles can be controlled. Electrostatic interaction of carboxylic groups on the NPs surface may be key factor in controlling the size and shape of the Au NPs. The Zeta potential measurement shows the stability of prepared Au nanoparticles at room temperature. The nonlinear optical parameters were calculated and the results confirm that the prepared gold nanoparticles could be used in optical applications such as optical switch and optical limiter.
Declarations
Author contribution statement
Pugazhendhi Shanmugam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Author of this manuscript Pugazhendhi Shanmugam would like to thank Prof.R.Jayavel, Centre for Nanoscience and Technology, Anna University and Prof. P.K.Palanisamy, Anna University for valuable suggestions and guidance in this work.
References
- 1.Yon J.N., Lead J.R. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008;400:396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 2.Duan H., Wang D., Li Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015;44:5778–5792. doi: 10.1039/c4cs00363b. [DOI] [PubMed] [Google Scholar]
- 3.Sabine S., Jolanda S., Rabah B. Surface plasmon resonance: signal amplification using colloidal gold nanoparticles for enhanced sensitivity. Rev. Anal. Chem. 2014;33:153–164. [Google Scholar]
- 4.Vincenzo A., Roberto P., Marco F., Onofrio M.M., Maria A.,I. Surface plasmon resonance in gold nanoparticles: a review. J. Phys. Condens. Matter. 2017;29 doi: 10.1088/1361-648X/aa60f3. [DOI] [PubMed] [Google Scholar]
- 5.Annika L., Yu P.B., Ulrich S., Willi J.D. Molecularly stabilised ultrasmall gold nanoparticles: synthesis, characterization and bioactivity. Nanoscale. 2013;5:6224–6242. doi: 10.1039/c3nr00916e. [DOI] [PubMed] [Google Scholar]
- 6.Yu J., Xu D., Guan H.N., Wang C., Huang L.K. Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Mater. Lett. 2016;166:110–112. [Google Scholar]
- 7.Rizzi V., Gubitosa J., Fini P., Nuzzo S., Agostiano A., Cosma P. Snail slime-based gold nanoparticles: an interesting potential ingredient in cosmetics as an antioxidant, sunscreen, and tyrosinase inhibitor. J. Photochem. Photobiol. B Biol. 2021 doi: 10.1016/j.jphotobiol.2021.112309. [DOI] [PubMed] [Google Scholar]
- 8.Xinping Z., Sun B., Richard H., Guo H., Nau D., Giessen H. Metallic photonic crystals based on solution-processible gold nanoparticles. Nano Lett. 2006;6:651–655. doi: 10.1021/nl052361o. [DOI] [PubMed] [Google Scholar]
- 9.Budnyk A.P., Damin A., Agostini G., Zecchina A. Gold nanoparticle aggregates immobilized on high surface area silica substrate for efficient and clean SERS applications. J. Phys. Chem. C. 2010;114:3857–3862. [Google Scholar]
- 10.Hurtado R.B., Cortez-Valadez M., Ramírez-Rodríguez L.P., Larios-Rodriguez E., A Alvarez R., Rocha-Rocha O., Flores-Acosta M. Instant synthesis of gold nanoparticles at room temperature and SERS applications. Phys. Lett. 2016;380:2658–2663. [Google Scholar]
- 11.Hammami I., Alabdallah N. Gold nanoparticles: synthesis properties and applications. J. King Saud Univ. Sci. 2021 [Google Scholar]
- 12.Punnoose M.S., Bijimol D., Mathew B. Microwave assisted green synthesis of gold nanoparticles for catalytic degradation of environmental pollutants. Environ. Nanotechnol. Monit. Manag. 2021;16 [Google Scholar]
- 13.Sengani M., Grumezescu A.M., Rajeswari V.D. Recent trends and methodologies in gold nanoparticle synthesis – a prospective review on drug delivery aspect. Open Nano. 2017;2:37–46. [Google Scholar]
- 14.Bennur T., Khan Z., Kshirsagar R., Javdekar V., Zinjarde S. Biogenic gold nanoparticles from the Actinomycete Gordonia amarae: application in rapid sensing of copper ions. Sensor. Actuator. B Chem. 2016;233:684–690. [Google Scholar]
- 15.Ahmed S., Ikram S. Biosynthesis of gold nanoparticles: a green approach. J. Photochem. Photobiol. B Biol. 2016;161:141–153. doi: 10.1016/j.jphotobiol.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 16.Biao L., Tan S., Meng Q., Gao J., Zhang X., Liu Z., Fu Y. Green synthesis, characterization and application of proanthocyanidins-functionalized gold nanoparticles. Nanomate. 2018;8:53. doi: 10.3390/nano8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dizman H.M., Kazancioglu E.O., Shigemune T., Takahara S., Arsu N. High sensitivity colorimetric determination of L-cysteine using gold nanoparticles functionalized graphene oxide prepared by photochemical reduction method. Spectrochim. Acta Part A: Mol. Biomole. Spec. 2021 doi: 10.1016/j.saa.2021.120294. [DOI] [PubMed] [Google Scholar]
- 18.Yu N., Bukharinova S., Khamzina E.I., Tarasov A.V., Vidrevich M.B., Brainina K.Z. The effect of the antioxidant activity of plant extracts on the properties of gold nanoparticles. Nanomaterials. 2019;9:1655. doi: 10.3390/nano9121655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam N.U., Jalil K., Shahid M., Rauf A., Muhammad N., Khan A., Shah M.R., Khan M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab. J. Chem. 2019;12:2914–2925. [Google Scholar]
- 20.Rahman M., Islam M. Alpinia calcarata Roscoe: a potential phytopharmacological source of natural medicine. Phcog. Rev. 2015;9:55–62. doi: 10.4103/0973-7847.156350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugazhendhi S., Kirubha E., Palanisamy P.K., Gopalakrishnan R. Synthesis and characterization of silver nanoparticles from Alpinia calcarata by Green approach and its applications in bactericidal and nonlinear optics. Appl. Surf. Sci. 2015;357:1801–1808. [Google Scholar]
- 22.Khandel P., Shahi S.K., Soni D.K., Yadaw R.K., Kanwar L. Alpinia calcarata: potential source for the fabrication of bioactive silver nanoparticles. Nano. Converg. 2018;5:37. doi: 10.1186/s40580-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhanga Y., Wang Y. Nonlinear optical properties of metal nanoparticles: a review. RSC Adv. 2017;7:45129–45144. [Google Scholar]
- 24.Fu Y., Rashid A.G., Krishnendu P.S., Zhou C., Rao K.S., Guo C. Size-dependent off-resonant nonlinear optical properties of gold nanoparticles and demonstration of efficient optical limiting. Opt. Mater. Express. 2019;9 [Google Scholar]
- 25.Patra J.K., Kwon Y., Baek K.H. Green biosynthesis of gold nanoparticles by onion peel extract: synthesis, characterization and biological activities. Adv. Powder Technol. 2016;27:2204–2213. [Google Scholar]
- 26.Hua Y., Chandra K., Dam D.H.M., Wiederrecht G.P., Odom T.W. Shape-dependent nonlinear optical properties of anisotropic gold nanoparticles. J. Phys. Chem. Lett. 2015;6:4904–4908. doi: 10.1021/acs.jpclett.5b02263. [DOI] [PubMed] [Google Scholar]
- 27.Rout A., Boltaev G.S., Ganeev R.A., Fu Y., Maurya S.K., Kim V.V., Guo C. Nonlinear optical studies of gold nanoparticle films. Nanomaterials. 2019;9:291. doi: 10.3390/nano9020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olesiak-Banska J., Gordel M., Kolkowski R., Matczyszyn K., Samoc M. Third-order nonlinear optical properties of colloidal gold nanorods. J. Phys. Chem. C. 2012;116:13731–13737. [Google Scholar]
- 29.Venkatesh M., Ganeev R.A., Ivanov D.S., Boltaev G.S., V Kim V., Liang J., Guo C. High-order harmonic generation in Au nanoparticle-contained plasmas. Nanomaterials. 2020;10:234. doi: 10.3390/nano10020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shokoufi N., Hajibaba S.N. The third-order nonlinear optical properties of gold nanoparticles-methylene blue conjugation. Opt Laser. Technol. 2019;112:198–206. [Google Scholar]
- 31.Pugazhendhi S., Palanisamy P.K., Jayavel R. Synthesis of highly stable silver nanoparticles through a novel green method using Mirabillis jalapa for antibacterial, nonlinear optical applications. Opt. Mater. 2018;79:457–463. [Google Scholar]
- 32.Pugazhendhi S., Sathya P., Palanisamy P.K., Gopalakrishnan R. Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 2016;159:155–160. doi: 10.1016/j.jphotobiol.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Paul B., Bhuyan B., Purkayastha D.D., Vadivel S., Dhar S.S. One-pot green synthesis of gold nanoparticles and studies of their anticoagulative and photocatalytic activities. Mater. Lett. 2016;185:143–147. [Google Scholar]
- 34.Teixeira P.R., Santos M.S., Silva A.L.G., Bao S.N., Azevedo R.B., Sales M.J.A., Paterno L.G. Photochemically-assisted synthesis of non-toxic and biocompatible gold nanoparticles. Colloids Surf. B Biointerfaces. 2016;148:317–323. doi: 10.1016/j.colsurfb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Patra S., Mukherjee S., Barui A.K., Ganguly A., Sreedhar B., Patra C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim N.A., Eid B.M., Abdel-Aziz M.S. Green synthesis of AuNPs for eco-friendly functionalization of cellulosic substrates. Appl. Surf. Sci. 2016;389:118–125. [Google Scholar]
- 37.Ghodake G., Kim D.Y., Jo J.H., Jang J., Lee D.S. One-step green synthesis of gold nanoparticles using casein hydrolytic peptides and their anti-cancer assessment using the DU145 cell line. J. Ind. Eng. Chem. 2016;33:185–189. [Google Scholar]
- 38.Hwang S.J., Jun S.H., Park Y., Cha S.H., Yoon M., Cho S., Park Y. Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation. Nanomed. Nanotechnol. Biol. Med. 2015;11:1677–1688. doi: 10.1016/j.nano.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Luo H., Huang Y., Lai K., Rasco B.A., Fan Y. Surface-enhanced Raman spectroscopy coupled with gold nanoparticles for rapid detection of phosmet and thiabendazole residues in apples. Food Control. 2016;68:229–235. [Google Scholar]
- 40.Zhang Y., Yang M., Portney N.G., Cui D., Budak G., Ozbay E., Ozkan C.S. Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices. 2008;10:321–328. doi: 10.1007/s10544-007-9139-2. [DOI] [PubMed] [Google Scholar]
- 41.Sheik-Bahae M., Said A.A., Wei T.H., Hagan D.J., Van Stryland E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quant. Electron. 1990;26:760–769. [Google Scholar]
- 42.El-Sadek M.A., Nooralden A.Y., Babu S.M., Palanisamy P.K. Influence of different stabilizers on the optical and nonlinear optical properties of CdTe nanoparticles. Opt Commun. 2011;284:2900–2904. [Google Scholar]
- 43.Geethakrishnan T., Palanisamy T.P.K. Z-scan determination of the third-order optical nonlinearity of a triphenylmethane dye using 633nm He–Ne laser. Optics Comm. 2007;270:424–428. [Google Scholar]
- 44.Chen H., Kou X., Yang Z., Ni W., Wang J. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir. 2008;24:5233–5237. doi: 10.1021/la800305j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.