Abstract
Background:
Brown algae have gained worldwide attention due to their significant biological activities, such as antidiabetic properties. In the present study, the antidiabetic properties of six brown algae from the Persian Gulf were investigated.
Methods:
An experimental study was conducted from 2017 to 2019 to examine the inhibitory effects of six brown algae against the α-glucosidase activity. Methanol (MeOH) and 80% MeOH extracts of Colpomenia sinuosa, Sargassum acinaciforme, Iyengaria stellata, Sirophysalis trinodis, and two accessions of Polycladia myrica were analyzed. The effect of 80% MeOH extracts of Sirophysalis trinodis on blood glucose levels in streptozotocin-induced diabetic rats was evaluated. Chemical constituents of brown algae were analyzed using thin-layer chromatography and liquid chromatography-mass spectrometry techniques. Data were analyzed using SPSS software, and P<0.05 was considered statistically significant.
Results:
The 80% MeOH extracts of Iyengaria stellata (IC50=0.33±0.15 μg/mL) and Colpomenia sinuosa (IC50=3.50±0.75 μg/mL) as well as the MeOH extracts of Colpomenia sinuosa (IC50=3.31±0.44 μg/mL) exhibited stronger inhibitory effect on α-glucosidase than the acarbose (IC50=160.15±27.52 μg/mL, P<0.001). The 80% MeOH extracts of Sirophysalis trinodis reduced postprandial blood glucose levels in diabetic rats compared to the control group (P=0.037). Fucoxanthin was characterized as the major antidiabetic agent in most of the algal extracts.
Conclusion:
Sirophysalis trinodis is recommended as a novel source for isolation and identification of potential antidiabetic compounds due to its high in vivo and in vitro antidiabetic effects.
Keywords: Diabetes mellitus, Streptozotocin, Blood glucose, Hypoglycemic agents, Alpha-glucosidase
What’s Known
Inhibiting α-glucosidase activity could retard the absorption of glucose and consequently control postprandial rise of blood glucose levels.
To date, no studies have investigated the inhibitory effect of brown algae from the Persian Gulf (Colpomenia sinuosa, Sargassum acinaciforme, Iyengaria stellata, Sirophysalis trinodis, and Polycladia myrica) on α-glucosidase activity.
What’s New
Colorimetric assay showed a strong inhibitory effect of 80% methanol extracts of Iyengaria stellata on α-glucosidase with the IC50 value of 0.33 µg/mL.
Administration of Sirophysalis trinodis extracts reduced postprandial blood glucose levels in diabetic rats compared to the control group.
Introduction
Type 2 diabetes mellitus is an abnormal rise in postprandial blood glucose levels, commonly known as postprandial hyperglycemia. One of the best treatments to control postprandial hyperglycemia is to inhibit carbohydrate hydrolyzing enzymes in the digestive system. In humans, salivary amylase initiates the degradation of starch into shorter oligomers. Then, pancreatic α-amylase completes hydrolysis by breaking down oligosaccharides into smaller units. Finally, intestinal α-glucosidases hydrolyze oligosaccharides into absorbable monosaccharides, such as glucose and fructose. 1 The α-Glucosidase enzyme plays a key role in catalyzing the hydrolytic cleavage of oligosaccharides into glucose molecules in the small intestine. Inhibiting α-glucosidase activity could retard the absorption of glucose and consequently control postprandial blood glucose levels. 2
The World Health Organization ranks diabetes among the top 10 causes of death and has predicted a 70% prevalence increase in 2030 versus 2000. 3 In addition to synthetic drugs, it is suggested that certain dietary habits (e.g., consuming algae as a source of nutrition) may lead to a reduction of postprandial blood sugar levels. 4 , 5 The antidiabetic effects of algae are attributed to their unsaturated fatty acids, polyphenolic compounds, and dietary fiber contents. 6 It has been reported that the antidiabetic properties of brown algae are due to their inhibitory effects on α-amylase and α-glucosidase. 7 , 8 To demonstrate this, the effect of two brown algae on insulin concentrations was clinically evaluated in diabetic men and women. It was shown that ingestion of 500 mg brown algae altered the modulation of insulin homeostasis after a meal, while it had no significant effects on plasma glucose levels. 9 Recently, the bioactive effect of marine natural products has attracted worldwide attention due to their significant antidiabetic, antibacterial, anticancer, antifungal, anti-inflammatory, and antiviral properties. 10 Different secondary metabolites have been found to be responsible for antidiabetic activity in algae, such as dieckol (a phlorotannin isolated from brown algae) and fucoidan (a sulfated polysaccharide). 11 , 12 Additionally, carotenoids are a group of secondary metabolites with antidiabetic properties affecting metabolic enzymes. 13
Previous studies reported various biological effects of different brown algae, such as Colpomenia sinuosa (C. sinuosa), Sargassum acinaciforme (Sar. acinaciforme), Iyengaria stellata (I. stellata), Sirophysalis trinodis (S. trinodis), and Polycladia myrica (P. myrica). It showed that the polysaccharide extracts of C. sinuosa exhibited potent cytotoxic and antioxidant activity. 14 An aqueous crude extract of C. sinuosa, collected from the coast of Lebanon, exhibited high antioxidant, anti-inflammatory, and fair antimicrobial activities. 15 Among the examined algal species in our study, samples of S. trinodis and P. myrica from the Persian Gulf inhibited α-amylase with inhibitory concentration (IC50) values of 0.42 mg/mL and 0.72 mg/mL, respectively. 16 Furthermore, another study evaluated ethanolic extracts of the Egyptian P. myrica using in vivo antidiabetic assays and suggested the alga’s ability to improve diabetes by reducing insulin resistance and glucose concentration. 17 A recent animal study has also reported the antidiabetic effect of phlorotannins, extracted from Cystoseira compressa, as it reduced glucose levels in rat serum. 18
In the present study, for the first time, the inhibitory effects of the above-mentioned algal extracts (collected from the coast of the Persian Gulf) on α-glucosidase were evaluated. Additionally, to examine their in vivo antidiabetic potential, we selected one of the active algae for further analysis using liquid chromatography-mass spectrometry (LC-MS) and thin-layer chromatography (TLC) techniques.
Materials and Methods
The present experimental study was conducted during 2017 to 2019 at the Medicinal and Natural Products Chemistry Research Center and Department of Pharmacology of Shiraz University of Medical Sciences, Shiraz, Iran.
Chemicals and Reagents
Acarbose, α-glucosidase (EC 3.2.1.20), streptozotocin (STZ), and p-nitrophenyl-α-D-glucopyranoside (PNPG) were purchased from Sigma-Aldrich Chemie GmbH (Germany), and all the solvents were purchased from Merck chemicals GmbH (Germany). Accu-Chek blood glucose meter was purchased from Roche Diagnostic GmbH (Germany). Analytical and preparative TLC experiments were carried out using Silica Gel 60 F254 glass plates (20×20 cm) with a gel film thickness of 0.25 mm (Merck, Germany). All other reagents were of analytical grade.
Algal Samples
Algal samples were collected in March 2015 from various locations along the Persian Gulf coastal region (Bushehr Province, Iran) (table 1). P. myrica was collected from two villages (Owli-ye-jonubi and Ziarat) along the coast of Bushehr. Collected algae were approved by a taxonomist (third author).
Table 1.
Details of the brown algae collected along the Persian Gulf coastal region, Bushehr, Iran
| Species | Source | Latitude and longitude | Voucher number |
|---|---|---|---|
| Colpomenia sinuosa | Shoghab park, Bushehr City | 28○54′51”N / 50○48′51”E | Pc-93-6-3-1.1 |
| Sargassum acinaciforme | Owli-ye-jonubi, Bushehr Province | 27○50′03”N / 51○53′55”E | Pc-93-6-2-1.1 |
| Iyengaria stellata | Ziarat, Bushehr Province | 28○10′32”N / 51○16′38”E | Pc-93-6-8-1.1 |
| Sirophysalis trinodis | Ziarat, Bushehr Province | 28○10′32”N / 51○16′38”E | Pc-93-6-9-1.1 |
| Polycladia myrica (1) | Owli-ye-jonubi, Bushehr Province | 27○50′03”N / 51○53′55”E | Pc-93-6-12-1.1 |
| Polycladia myrica (2) | Ziarat, Bushehr Province | 28○10′32”N / 51○16′38”E | Pc-93-6-12-1.2 |
Preparation of Algal Extracts
Maceration was used to obtain extracts with two successive solvents, methanol (MeOH) and 80% MeOH, at room temperature for two days. The resulting extracts were concentrated at ≥40 ºC under reduced pressure.
Liquid Chromatography (LC), Diode Array Detection (DAD), Electrospray Ionization (ESI), and Mass Spectrometry (MS) Conditions
The algal extracts were analyzed using Shimadzu LCMS-2010EV (Kyoto, Japan), equipped with an ESI mass ionization source (±) and an SPD-M20A DAD. A Shim-Pack XR-ODS column (2×50 mm) was used with the mobile phase at a flow rate of 0.25 mL/min. The mobile phase included MeOH and water (9:1) running for 20 min. The LCMS was injected with 5 μL of the sample solutions (1 mg/mL). The MS analytical parameters were MS detector voltage ±1.5 KV, curved desolvation line (CDL) voltage ±10 V, interface voltage ±4.5 KV, Q-array RF voltage ±150 V, scanned mass spectra range of m/z 100-1000 D, and nebulizer gas (N2) with a flow rate of 1.5 L/min. Finally, the heat block and CDL temperatures were set at 230 °C and 275 °C, respectively. The DAD-UV spectra were recorded at a wavelength (λ) of 190-600 nm.
α-Glucosidase Activity
The α-glucosidase inhibitory activity of MeOH and 80% MeOH algal extracts was measured using the modified colorimetric method as described in a previous study. 19 Briefly, 5 μL of algal extract or acarbose solution at different concentrations was added to a 20 μL α-glucosidase solution (0.25 U/mL) in 0.1 M potassium phosphate buffer (pH 6.8) followed by 90 μL of the buffer. Then, the mixture was incubated at 37 ºC in the dark for 10 min in 96-well microplates. In the next step, 15 μL of PNPG solution (2.5 mM) of the buffer was added to the incubated solution and further incubated at 37 ºC for an additional 30 min. Finally, 80 μL of 0.2 M Na2CO3 was added to each well to stop the reaction. Then, the absorbance of the solution in each well (A) was measured at λ=405 nm using a microplate reader (model 680, Bio-Rad Laboratories, Inc.). The control solution contained 5 μL buffer solution instead of the extracts or standard drug. The inhibition percentage of α-glucosidase was calculated using the following formula.
where Acontrol is the absorbance of the control and Aextract is the absorbance of the sample.
IC50 (concentration of the samples that inhibited 50% of the enzyme activity) was calculated from a linear correlation between the %inhibition and their respective concentration using CurveExpert 1.4 software (Hyams Development, Chattanooga, TN, USA). The kinetic of the enzyme was evaluated by drawing a Lineweaver-Burk plot of one of the potent α-glucosidase inhibitors from the algal extracts measured at different substrate concentrations of PNPG (1–10 mM). 20 , 21
Animals and Ethical Guidelines
A total of 30 healthy adult male Sprague-Dawley rats weighing 180-220 g were obtained from the Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences (Shiraz, Iran). Animals were fed a standard rodent diet and water, and kept in a regime of a light-dark (12:12 hour) cycle. The animal experiments were carried out in full accordance with the guidelines published by the Iran National Committee for Ethics in Biomedical Research. 22 The study was approved by the local Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.REC.1399.279).
Induction of Diabetes
After seven days of the quarantine period and 10 hours of fasting before injection, diabetes was induced in animals through a single intraperitoneal injection of 50 mg/Kg of STZ. The STZ solution in citrate buffer (0.1 M, pH 4.5) was prepared fresh before injection. Five days after STZ injection, a blood sample of each animal was taken from the tail vein and plasma glucose concentrations were measured using the glucose meter. Rats with blood sugar levels higher than 300 mg/dL (more than 90% of injected animals) were considered as diabetics. 23 No exogenous insulin treatment was given. 24
Measurement of Blood Glucose Levels
STZ-induced diabetic rats fasted overnight and were randomly divided into three groups, namely diabetic controls (received deionized water), positive controls (treated with acarbose (30 mg/Kg) as a standard antidiabetic drug), and diabetic rats (received 80% MeOH extract of S. trinodis, 30 mg/Kg). Half an hour after the above-mentioned treatments, the rats were orally administrated a sucrose solution (2 g/Kg). The plasma glucose samples were prepared at 0, 30, 60, 90, and 120 min after the sucrose administration, as described in a previous study. 25 The blood glucose levels of the above samples were measured using the glucose meter.
TLC Analysis
The MeOH and 80% MeOH extracts of all the algae were analyzed using silica gel TLC and optimized separation of various mobile phases.
Statistical Analysis
The data of more than nine replicates were expressed as mean±SEM (standard error of measurment). Microsoft Excel 2016 software (Microsoft Corporation, USA) was used for statistical analysis, and IC50 values were evaluated using CurveExpert 1.4 software. The data were analyzed using SPSS software version 16.0 (SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) followed by Dunnett’s and Tukey’s tests was performed to evaluate the difference between different datasets and their significance. P values less than 0.05 were considered statistically significant.
Results
In Vitro α-Glucosidase Inhibition
The inhibitory effect of algal extracts against α-glucosidase was determined using PNPG as the substrate in a colorimetric assay. Maximum α-glucosidase inhibition was measured for the 80% MeOH extract of I. stellata with IC50=0.33 µg/mL. Other extracts had IC50 values ≤30 µg/mL, namely C. sinousa (IC50=3.31 µg/mL for MeOH and IC50=3.50 µg/ml for 80% MeOH extract), P. myrica (2) (IC50=18.83 µg/mL for MeOH extract), S. trinodis (IC50=25.38 µg/mL for 80% MeOH extract), and Sar. acinaciforme (IC50=28.06 µg/mL for 80% MeOH extract) (table 2). The MeOH extract of P. myrica (1) had the weakest enzyme inhibition potential (IC50>1000 µg/mL). Acarbose was used as a standard inhibitor and exhibited an IC50 value of 160.15 µg/mL. The results showed that most of the tested algae had significant inhibition versus the acarbose (P<0.001).
Table 2.
IC50 values of α-glucosidase inhibition of the algal extracts and acarbose
| Algae | Enzyme inhibition IC50 (µg/ml)* Mean±SEM |
|---|---|
| C. sinousa (MeOH) | 3.31±0.44a |
| C. sinousa (80% MeOH) | 3.50±0.75a |
| Sar. acinaciforme (80% MeOH) | 28.06±3.88b |
| Sar. acinaciforme (MeOH) | 316.25±47.25e |
| I. stellata (MeOH) | 114.64±20.25d |
| I. stellata (80% MeOH) | 0.33±0.15a |
| S. trinodis (80% MeOH) | 25.38±4.36b |
| S. trinodis (MeOH) | 133.90±34.94d |
| P. myrica (1) (MeOH) | >1000 |
| P. myrica (1) (80% MeOH) | 232.82±29.83e |
| P. myrica (2) (MeOH) | 18.83±2.29b |
| P. myrica (2) (80% MeOH) | 49.88±2.70c |
| Acarbose (standard) | 160.15±27.52d |
*IC50 values were calculated using linear regression and their significance difference (P values) were compared using one-way ANOVA-Tukey. Same letters represent no significant differences (P>0.05) between the IC50 values, whereas values with different letters have P<0.05 (i.e., statistically significant). aP=0.80-1, bP=0.29-0.98, dP=0.62-0.54, eP=0.23; P value between groups: a and b (P=0.00-0.01), b and c (P=0.001-0.008), c and d (P=0.000-0.009), d and e (P=0.007-0.023). Values are expressed as mean±SEM of three replicates. At least five serially diluted solutions of each extract were tested to calculate the IC50 values. Different letters represent significant differences (P<0.05).
α-Glucosidase Inhibition Kinetics
A Lineweaver-Burk plot of α-glucosidase inhibitory activity of the 80% MeOH extract of S. trinodis at 0 and 1.2 mg/mL stock solution and at different PNPG concentrations (1–10 mM) was drawn (figure 1). The results showed that an increase in extract concentration did not affect Km, and it remained about 2.7 mM, whereas Vmax decreased by 0.09 and 0.02 mM/min, respectively. This indicated that the extract inhibited α-glucosidase in a non-competitive manner.
Figure 1.
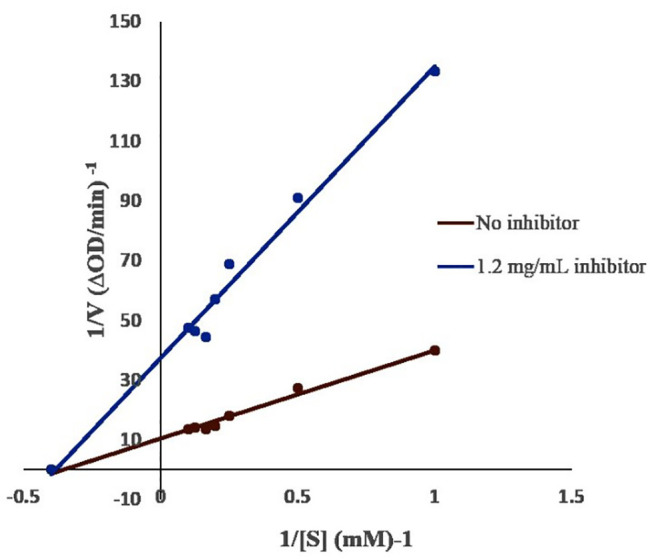
Lineweaver-Burk plot analysis of the kinetics of α-glucosidase inhibition measured in the presence of 0 and 1.2 mg/mL of 80% MeOH S. trinodis extract. α-Glucosidase=0.25 U/mL and pH 6.8 at room temperature.
In Vivo Bioassay
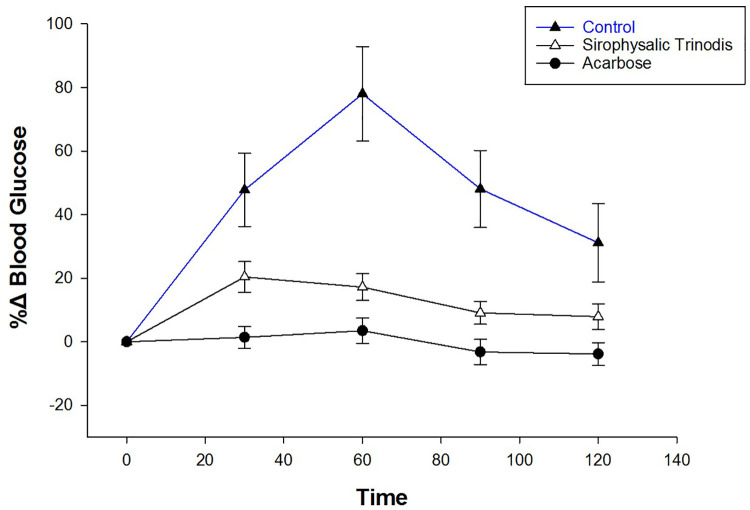
Since the majority of algal extracts exhibited high enzyme inhibition, the 80% MeOH extract of S. trinodis was selected for in vivo tests on STZ-induced diabetic rats. The selection was made based on its medium inhibition potential and enough extract to perform the tests. The effect of the alga on blood glucose levels of STZ-induced diabetic rats was investigated after a meal. Postprandial blood glucose levels of rats administered algal extract were lower than those of the control group (figure 2). The blood glucose levels increased up to 78% at 60 min after sucrose administration and decreased thereafter. When the STZ-induced diabetic rats were administrated with algal extracts, the upward trend of the percentage change in postprandial blood glucose levels was significantly reduced at 30, 60, 90, and 120 min with a percentage change of 20.38±4.83, 17.2±4.21, 9.1±0.58, and 7.9±3.97%, respectively (P=0.05 for the blood glucose level at 30 min compared to levels at 90 and 120 min). Such a decrease in blood glucose levels indicates that S. trinodis could retard carbohydrate digestion.
Figure 2.
The percentage change (%∆) of blood glucose levels was calculated after administration of 80% MeOH extract of S. trinodis in STZ-induced diabetic rats. Control (deionized water), S. trinodis extract (30 mg/Kg), and acarbose (30 mg/Kg) were co-administered orally with sucrose (2 g/Kg). Data are expressed as mean±SEM.
The results showed that the percentage change of relative blood glucose level at 60 minutes after sucrose administration was significantly different for the acarbose and algal treated mice compared to the control group (P=0.007 and P=0.037, respectively). Whereas, the values obtained for the other groups were similar (P=0.783) (table 3).
Table 3.
Statistical analysis of the differences in blood glucose levels between the control, acarbose, and algal-treated rats
| Treated rats (I) | Treated rats (J) | Blood glucose level (mg/dL) mean±SEM | P value | 95% Confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Control | Acarbose | 192.97±59.57 | 0.007 | 47.15 | 338.78 |
| S. trinodis (80% MeOH) | Acarbose | 43.48±56.65 | 0.783 | -95.18 | 182.14 |
| S. trinodis (80% MeOH) | Control | -149.48±58.27 | 0.037 | -291.66 | -7.31 |
| Acarbose | Control | -192.97±59.57 | 0.007 | -338.30 | -47.63 |
Data were calculated using one-way analysis of variance (ANOVA) followed by post-hoc Dunnett’s test (dependent variable: time point at 60 min). Blood glucose levels are expressed as mean±SEM.
TLC Analysis
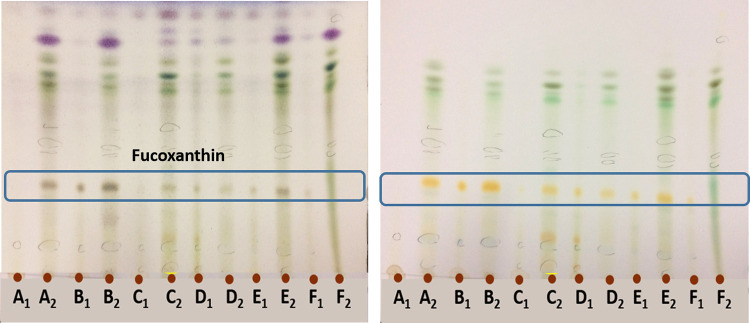
Fingerprint analysis was performed on silica gel TLC plates to detect chemical constituents of the algal extract. Orange spots with the retention factor (Rf) of about 0.46 were present in most of the extracts, identified as fucoxanthin. The compound was then separated using preparative TLC. The best TLC solvent system was developed using petroleum ether:ethyl acetate:acetic acid (6:1:1) (figure 3).
Figure 3.
Silica gel TLC chromatograms were eluted with petroleum ether:ethyl acetate:acetic acid (6:1:1). The left chromatogram was generated after spraying with vanillin sulfuric acid reagent and heated at 110 ºC. The right chromatogram was observed in daylight. The 80% MeOH and MeOH algal extracts from left to right are S. trinodis (A1, A2), Sar. acinaciforme (B1, B2), C. sinuosa (C1, C2), I. stellata (D1, D2), P. myrica (2) (E1 E2), P. myrica (1) (F1, F2), respectively.
LC-MS Analysis
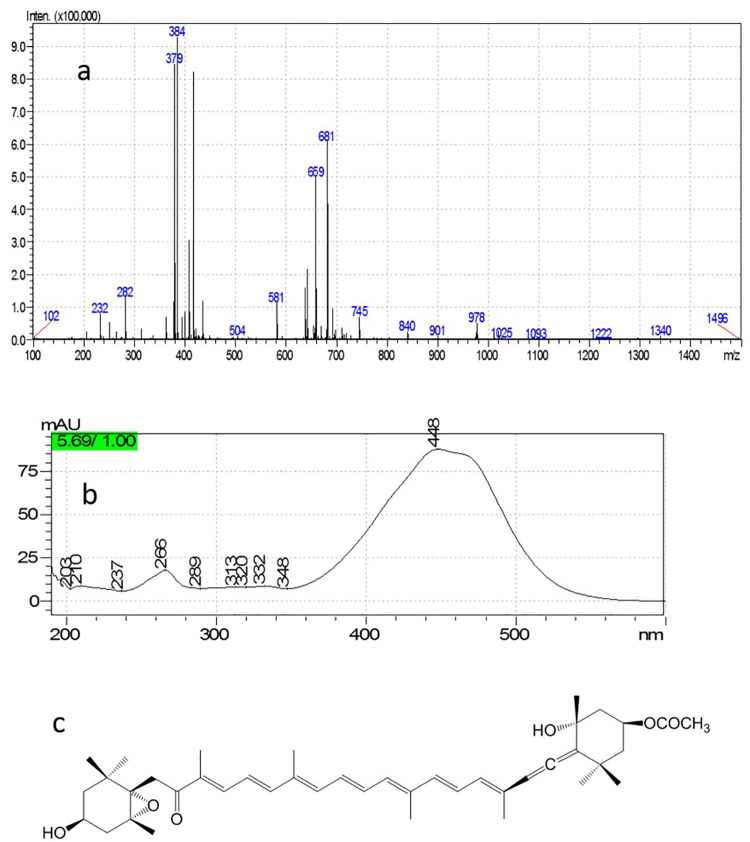
The LC-DAD-ESI-MS technique was used to characterize the chemical structures of isolated compounds separated by preparative TLC. ESI-MS was carried out in positive ion mode, and two quasi molecular and daughter ions were detected at m/z 659 ([M+H]+, 681 [M+Na]+, 641 [M+H-H2O]+, and 581 [M+H-H2O-AcOH]+. The UV spectrum exhibited λmax=448 nm, which is comparable with those reported for fucoxanthin (figure 4).
Figure 4.
ESI-MS (a) and PDA-UV (b) spectra are recorded for fucoxanthin (c).
Discussion
Among the examined algal extracts, C. sinuosa, Sar. acinaciforme, I. stellata, and S. trinodis exhibited a strong inhibitory effect on α-glucosidase activity. S. trinodis was selected for in vivo testing. After the administration of algal extracts, the results showed reduced blood glucose levels in diabetic rats compared to the control group. Furthermore, fucoxanthin from S. trinodis was isolated and identified through its ESI-MS and PDA-UV spectra, and then the data were compared with those published in the literature. 26 , 27
Brown algae have attracted the attention of many researchers due to their antidiabetic properties. For instance, the inhibitory effect of MeOH extract of S. trinodis (IC50=0.42 mg/mL) and ethyl acetate extract of P. myrica (IC50=0.72 mg/mL) on α-amylase 16 was in accordance with their stronger inhibitory effects on α-glucosidase activity (low IC50 values in table 2). This suggests the effect of solvent polarity in extracting active antidiabetic agents. Their use is thus recommended in future research, since compounds with strong inhibitory activity against α-glucosidase, more than α-amylase, has superior antidiabetic effect, as it lowers absorbable monosaccharide levels in the gut. 28 Among the reported antidiabetic properties of algal species, similar to those in our study, a methanolic extract (1 mg/mL) of the brown seaweed C. sinuosa (collected from the Korean island of Jeju) showed α-glucosidase inhibitory activity of >90%. 29 In another study, ethanolic extract of Sargassum serratifolium, from the coast of Busan in South Korea, was reported to inhibit α-glucosidases with an IC50 value 24.16±0.31 μg/mL compared to a positive control acarbose (108.74±2.96 μg/mL). 30 A recent study reported that an ethanolic extract of the Egyptian P. myrica lowered postprandial glucose levels in alloxan-induced diabetic rats. 17
In a previous study, the inhibitory effect of five edible brown and red algae on α-glucosidase activity was investigated, among which acetone extract of Undaria pinnatifida was reported to have the strongest effect with IC50=0.08±0.002 mg/mL. 31 Brown algae contain better antidiabetic nutraceuticals than red algae. Fucoxanthin, the main carotenoid in brown algae, has been shown to have many biological functions. 32 HPLC-guided purification of the algal extract showed fucoxanthin as an active α-glucosidase inhibitor agent with an IC50 value of 0.047±0.001 mg/mL and a mixed-type inhibition mechanism. 31 In an animal study, fucoxanthin was reported to significantly improve insulin resistance, blood glucose level, and lipid concentration in mice. Therefore, fucoxanthin is recommended as a potential functional food and suitable drug for type 2 diabetes mellitus. 33
The MeOH extracts of the two accessions of P. myrica (collected from the villages Owli-ye-jonubi and Ziarat along the coastal area of Bushehr) showed different α-glucosidase inhibitory activities, depending on the location, where the samples were collected. In addition, most of the aqueous 80% MeOH extracts exhibited better activity than those of the MeOH extracts. It seems they have various secondary metabolites, responsible for the biological activity due to their local adaptation or genotype. 34 Varying amounts of fucoxanthin were detected in almost all algal extracts in our study (figure 3). It has many biological activities including anticancer, anticholesterol, anti-inflammatory, and antidiabetic properties as well as inhibiting tumor cell growth. 35 , 36 Fucoxanthin has attracted the attention of researchers and industries for its application in food and cosmetic products. 37 In addition to its potential to inhibit α-glucosidase, fucoxanthin (isolated from edible brown algae such as Eisenia bicyclis and Undaria pinnatifida) inhibits different enzymes in diabetes pathways such as rat lens aldose reductase (RLAR), human recombinant aldose reductase (HRAR), advanced glycation end-product (AGE) formation, and protein tyrosine phosphatase 1B (PTP1B). 38 A previous study showed that fucoxanthin exhibited strong inhibitory activity against α-amylase and α-glucosidase (with IC50 values of 0.68 and 4.75 mmol/L, respectively) and suggested its usefulness in preventing diabetes. 39 In addition to fucoxanthin, based on in vivo and in vitro studies, phenolics and carbohydrates such as fucoidan from different brown algae are reported to exhibit major antidiabetic activity. 40 , 41
S. trinodis extract and fucoxanthin seem to have different enzyme inhibition mechanisms. This difference could be due to an inadequate amount of fucoxanthin measured in S. trinodis extract (see A1 in figure 3) and the presence of other bioactive compounds in the extract. Further phytochemical investigations are required to confirm the presence of other antidiabetic secondary metabolites. In addition to issues related to phytochemical analysis, there are other challenges involved in developing drugs from algal extracts. Overcoming the limited availability of algae and the quality of their extracts to obtain sufficient material for clinical studies is of great importance.
Conclusion
For the first time, we report a strong inhibitory effect against α-glucosidase activity by six brown algae obtained from the Persian Gulf coastal region, namely, C. sinuosa, Sar. acinaciforme and I. stellata, S. trinodis, and two accessions of P. myrica. Considering their inhibitory potential against α-glucosidase and α-amylase. 16 Further studies are recommended to confirm these algae as a suitable candidates for the development of diabetes drugs. Among these, S. trinodis and P. myrica 17 lowered postprandial blood glucose levels in diabetic rats. Fucoxanthin, as an effective antidiabetic nutraceutical, was detected in most algal extracts. However, other secondary metabolites could also be responsible for the marked antidiabetic activity of the studied algae. Further studies are recommended to identify additional bioactive agents, phenolic or polysaccharides, especially from aqueous methanol extracts of S. trinodis and Sar. acinaciforme, and methanol extracts of P. myrica. These algae are suggested as a valuable source for further isolation and identification of active antidiabetic compounds. However, it requires multidisciplinary collaboration for the cultivation of bioactive algae, exploring their potential as food products, and subsequent extraction and purification on a semi-industrial scale.
Acknowledgement
The study was financially supported by the National Institutes for Medical Research Development (NIMAD), Tehran, Iran (grant number: 971323). We would like to thank Ms Purkhosrow for her technical assistance with the in vivo experiments at the Department of Pharmacology, Shiraz University of Medical Sciences, Shiraz, Iran.
Authors’ Contribution
N.M: Designed and performed all of the experiments, analysed and interpreted the data; A.R.J and H.M: Were the supervisors of the project and design and involved in chemical, biochemical and biological analyses, and interpretation of the results; J.S: Is the algal taxonomist and characterized the organisms. All authors contributed in drafting and critically revision of the manuscript. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
None declared.
References
- 1.Truscheit E, Frommer W, Junge B, Müller L, Schmidt DD, Wingender W. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angewandte Chemie International Edition in English. 1981;20:744–61. doi: 10.1002/anie.198107441. [DOI] [Google Scholar]
- 2.Lebovitz HE. alpha-Glucosidase inhibitors. Endocrinol Metab Clin North Am. 1997;26:539–51. doi: 10.1016/s0889-8529(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Kim HC, Vitek L, Nam CM. Algae consumption and risk of type 2 diabetes: Korean National Health and Nutrition Examination Survey in 2005. J Nutr Sci Vitaminol (Tokyo) 2010;56:13–8. doi: 10.3177/jnsv.56.13. [DOI] [PubMed] [Google Scholar]
- 5.Tanemura Y, Yamanaka-Okumura H, Sakuma M, Nii Y, Taketani Y, Takeda E. Effects of the intake of Undaria pinnatifida (Wakame) and its sporophylls (Mekabu) on postprandial glucose and insulin metabolism. J Med Invest. 2014;61:291–7. doi: 10.2152/jmi.61.291. [DOI] [PubMed] [Google Scholar]
- 6.Sharifuddin Y, Chin YX, Lim PE, Phang SM. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar Drugs. 2015;13:5447–91. doi: 10.3390/md13085447. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayapu L, Chakraborty K, Valluru L. Marine Algae as a Potential Source for Anti-diabetic Compounds - A Brief Review. Curr Pharm Des. 2021;27:789–801. doi: 10.2174/1381612826666200909124526. [DOI] [PubMed] [Google Scholar]
- 8.Nazarudin MF, Isha A, Mastuki SN, Ain NM, Mohd Ikhsan NF, Abidin AZ, et al. Chemical Composition and Evaluation of the alpha-Glucosidase Inhibitory and Cytotoxic Properties of Marine Algae Ulva intestinalis, Halimeda macroloba, and Sargassum ilicifolium. Evid Based Complement Alternat Med. 2020;2020:2753945. doi: 10.1155/2020/2753945. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paradis ME, Couture P, Lamarche B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl Physiol Nutr Metab. 2011;36:913–9. doi: 10.1139/h11-115. [DOI] [PubMed] [Google Scholar]
- 10.Mayer AMS, Guerrero AJ, Rodriguez AD, Taglialatela-Scafati O, Nakamura F, Fusetani N. Marine Pharmacology in 2016-2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar Drugs. 2021;19 doi: 10.3390/md19020049. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Jeon YJ. Efficacy and safety of a dieckol-rich extract (AG-dieckol) of brown algae, Ecklonia cava, in pre-diabetic individuals: a double-blind, randomized, placebo-controlled clinical trial. Food Funct. 2015;6:853–8. doi: 10.1039/c4fo00940a. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Gao L, Zhou H, Ai C, Huang X, Wang M, et al. Opportunities and challenges of algal fucoidan for diabetes management. Trends in Food Science & Technology. 2021;111:628–41. doi: 10.1016/j.tifs.2021.03.028. [DOI] [Google Scholar]
- 13.Wang N, Manabe Y, Sugawara T, Paul NA, Zhao J. Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem. 2018;242:247–55. doi: 10.1016/j.foodchem.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 14.Matloub AA, Elsouda SSM, El-Senousy WM, Hamed M, Aly H, Ali SA, et al. In vitro antiviral, cytotoxic, antioxidant and hypolipidemic activities of polysaccharide isolated from marine algae. Int J Pharmacogn Phytochem Res. 2015;7:1099–111. [Google Scholar]
- 15.Al Monla RM, Dassouki ZT, Gali-Muhtasib H, Mawlawi HR. Chemical analysis and biological potentials of extracts from Colpomenia sinuosa. Pharmacognosy Research. 2020;12:272. [Google Scholar]
- 16.Pirian K, Moein S, Sohrabipour J, Rabiei R, Blomster J. Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. Journal of Applied Phycology. 2017;29:3151–9. doi: 10.1007/s10811-017-1152-0. [DOI] [Google Scholar]
- 17.Alwaleed EA, Jillany A, Kasem NR, Galal H. Evaluation of the pancreatoprotective effect of algal extracts on Alloxan-induced diabetic rat. Bioactive Carbohydrates and Dietary Fibre. 2020;24:100237. doi: 10.1016/j.bcdf.2020.100237. [DOI] [Google Scholar]
- 18.Gheda S, Naby MA, Mohamed T, Pereira L, Khamis A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ Sci Pollut Res Int. 2021;28:22886–901. doi: 10.1007/s11356-021-12347-5. [DOI] [PubMed] [Google Scholar]
- 19.Sheliya MA, Rayhana B, Ali A, Pillai KK, Aeri V, Sharma M, et al. Inhibition of alpha-glucosidase by new prenylated flavonoids from euphorbia hirta L. herb. J Ethnopharmacol. 2015;176:1–8. doi: 10.1016/j.jep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Lineweaver H, Burk D. The determination of enzyme dissociation constants. Journal of the American chemical society. 1934;56:658–66. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- 21.Daub CD, Mabate B, Malgas S, Pletschke BI. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, alpha-glucosidase. Int J Biol Macromol. 2020;151:412–20. doi: 10.1016/j.ijbiomac.2020.02.161. [DOI] [PubMed] [Google Scholar]
- 22. Iran National Committee for Ethics in Biomedical Research. [cietd 17 September 2021] Available from: https://ethics.research.ac.ir/IndexEn.php .
- 23.Leiter EH. Selecting the “right” mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol. 2009;560:1–17. doi: 10.1007/978-1-59745-448-3_1. [DOI] [PubMed] [Google Scholar]
- 24.Minaiyan M, Ghannadi A, Movahedian A, Hakim-Elahi I. Effect of Hordeum vulgare L. (Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res Pharm Sci. 2014;9:173–8. [ PMC Free Article ] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J. Effect of Rhemanniae radix on the hyperglycemic mice induced with streptozotocin. Journal of The Korean Society of Food Science and Nutrition. 2004;33:1133–8. doi: 10.3746/jkfn.2004.33.7.1133. [DOI] [Google Scholar]
- 26.Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH, Chung D, et al. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol. 2012;166:1843–55. doi: 10.1007/s12010-012-9602-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Wu H, Wen H, Fang H, Hong Z, Yi R, et al. Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Mar Drugs. 2015;13:6521–36. doi: 10.3390/md13106521. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akata I, Zengin G, Picot C, Mahomoodally M. Enzyme inhibitory and antioxidant properties of six mushroom species from the Agaricaceae family. South African Journal of Botany. 2019;120:95–9. doi: 10.1016/j.sajb.2018.01.008. [DOI] [Google Scholar]
- 29.Ko S-C, Lee S-H, Kang S-M, Ahn G, Cha S-H, Jeon Y-J. Evaluation of α-glucosidase Inhibitory Activity of Jeju Seaweeds Using High Throughput Screening (HTS) Technique. Journal of Marine Bioscience and Biotechnology. 2011;5:33–9. doi: 10.15433/KSMB.2011.5.4.033. [DOI] [Google Scholar]
- 30.Ali MY, Kim DH, Seong SH, Kim HR, Jung HA, Choi JS. alpha-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitory Activity of Plastoquinones from Marine Brown Alga Sargassum serratifolium. Mar Drugs. 2017;15 doi: 10.3390/md15120368. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaharudin N, Staerk D, Dragsted LO. Inhibition of alpha-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019;270:481–6. doi: 10.1016/j.foodchem.2018.07.142. [DOI] [PubMed] [Google Scholar]
- 32.Mikami K, Hosokawa M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int J Mol Sci. 2013;14:13763–81. doi: 10.3390/ijms140713763. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Xu W, Huang X, Zhao Y, Ren Q, Hong Z, et al. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. Journal of functional foods. 2018;48:515–24. doi: 10.1016/j.jff.2018.07.048. [DOI] [Google Scholar]
- 34.Gaubert J, Payri CE, Vieira C, Solanki H, Thomas OP. High metabolic variation for seaweeds in response to environmental changes: a case study of the brown algae Lobophora in coral reefs. Sci Rep. 2019;9:993. doi: 10.1038/s41598-018-38177-z. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu SJ, Liou CJ, Chen YL, Cheng SC, Huang WC. Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice. Cells. 2021;10 doi: 10.3390/cells10061311. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Zhao J, Fang C, Cao Q, Xing M, Li X, et al. Advances in Studies on the Pharmacological Activities of Fucoxanthin. Mar Drugs. 2020;18 doi: 10.3390/md18120634. [ PMC Free Article ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lourenço-Lopes C, Fraga-Corral M, Jimenez-Lopez C, Carpena M, Pereira A, Garcia-Oliveira P, et al. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends in Food Science & Technology. 2021;117:163–81. doi: 10.1016/j.tifs.2021.03.012. [DOI] [Google Scholar]
- 38.Jung HA, Islam M, Lee CM, Jeong HO, Chung HY, Woo HC, et al. Promising antidiabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclis and Undaria pinnatifida. Fisheries Science. 2012;78:1321–9. doi: 10.1007/s12562-012-0552-y. [DOI] [Google Scholar]
- 39.Kawee-Ai A, Kim AT, Kim SM. Inhibitory activities of microalgal fucoxanthin against α-amylase, α-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes. Journal of Oceanology and Limnology. 2019;37:928–37. doi: 10.1007/s00343-019-8098-9. [DOI] [Google Scholar]
- 40.Shan X, Liu X, Hao J, Cai C, Fan F, Dun Y, et al. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. 2016;82:249–55. doi: 10.1016/j.ijbiomac.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Y, Zhang J, Fan J, Clark J, Shen P, Li Y, et al. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on alpha-amylase, alpha-glucosidase, pancreatic lipase and tyrosinase. Food Res Int. 2018;113:288–97. doi: 10.1016/j.foodres.2018.07.021. [DOI] [PubMed] [Google Scholar]