Figure 2. STK25 binds to and phosphorylates PRKAR1A.
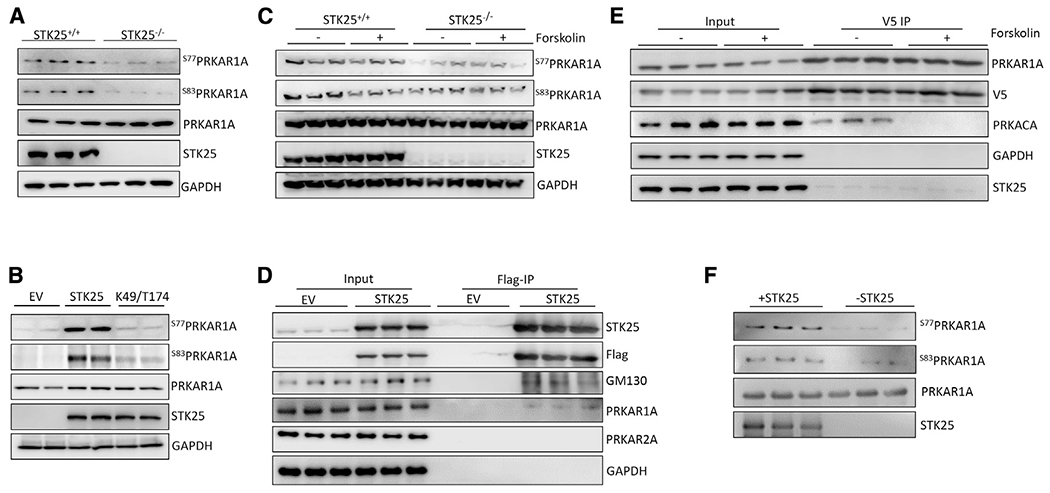
(A) Immunoblot of PRKAR1A phospho-S77 and -S83 in STK25+/+ and STK25−/− cardiomyocyte protein lysates. n = 3 for each condition.
(B) Immunoblots of phosphor-S77 and -S83 of PRKAR1A in STK25−/− cardiomyocytes transfected with empty vector (EV), wild-type STK25, and kinase dead K49R/T174A STK25. n = 2 for each condition.
(C) Forskolin (10 μM, 30 min)-stimulated STK25+/+ and STK25−/− cardiomyocytes immunoblotted for phosphorylation of PRKAR1A. n = 3 for each condition.
(D) Immunoprecipitation of FLAG-STK25 expressed in HEK293T cells and immunoblotted for PRKAR1A, PRKA2A, and GM130 (positive control binding partner). n = 3 for each condition.
(E) Co-immunoprecipitation of PRKAR1A-V5 with STK25 and PRKACA in HEK293T cells treated with forskolin (10 μM, 30 min). n = 3 for each condition.
(F) In vitro kinase assay of purified STK25 and PRKAR1A, immunoblotted for phosphorylation of S77 and S83 of PRKAR1A. n = 3 for each condition.
See also Figure S1.
