Figure 1.
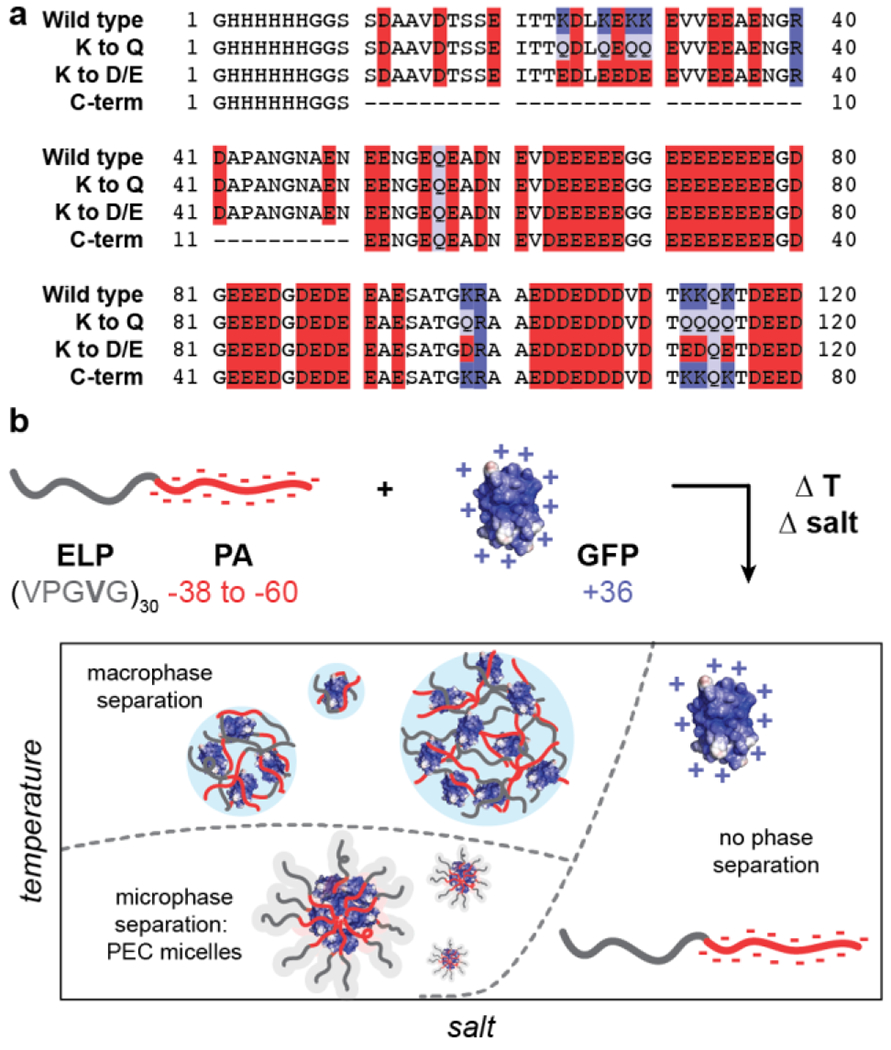
Schematic of prothymosin alpha (PA) variants studied here, (a) Primary amino acid sequence of the four PA variants with acidic residues (D,E) highlighted in red, basic residues (K, R) highlighted in blue, and glutamine residues (Q) highlighted in purple, (b) Fusion of a charge neutral elastin-like polypeptide (ELP) to the N-terminus of the PA variants enables rich phase behavior when complexed with oppositely charged polycationic proteins. A schematic of the phase behavior as a function of salt and temperature is shown.
