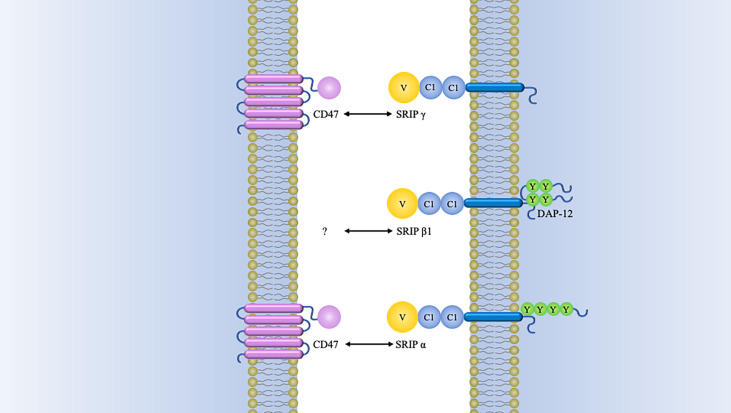
Figure 2.
Schematics of structure of SIRP family members. Besides SIRPα, SIRPβ1, and SIRPγ have also been identified in humans. Both of SIRPβ1 and SIRPγ consist of three Ig-like loops in their extracellular domains. SIRPβ1 is characterized by a basic amino acid side chain in its transmembrane domain with a very short cytoplasmic region. This transmembrane region is indispensable for binding of DAP12 (DNAX activation protein 12). It has been established that SIRPβ1 can mobilize the tyrosine kinase Syk, which was followed by MAPK (mitogen-activated protein kinase) activation and microglial phagocytosis enhancement (26). However, it remains unknown what the extracellular ligand for SIRPβ1 and how it might regulate cellular function. There is also a short cytoplasmic region in SIRPγ, but it is quite different from SIRPβ1. The former lacks a charged amino acid residue in its transmembrane region. Nevertheless, SIRPγ can still interact with CD47 by the way of protein-protein binding studies (27). One study has demonstrated that endothelial cell CD47 interacting with SIRPγ plays an important role in T-cell trans-endothelial migration (28). SIRPβ2 is expressed by cells of the monocyte-macrophage lineage and presumably has an association with DAP12 or a similar adaptor (14). SIRPδ has only one domain and has not yet been found any obvious means of membrane attachment (10).