Abstract
Rationale
Moderate to severe asthma is associated with impaired asthma control and quality of life (QoL) despite access to specialist care and modern pharmacotherapy. Breathing exercises (BrEX) improve QoL in incompletely controlled mild asthma, but impact in moderate to severe asthma is unknown.
Objectives
To investigate the effectiveness of BrEX as adjuvant treatment on QoL in patients with uncontrolled moderate to severe asthma.
Methods
Adult patients with incompletely controlled asthma attending respiratory specialist clinics were randomized to usual specialist care (UC) or UC and BrEX (UC + BrEX) with three individual physiotherapist-delivered sessions and home exercises. Primary outcome was asthma-related QoL (Mini-Asthma Quality of Life Questionnaire [Mini-AQLQ]) at 6 months on the basis of intention-to-treat analysis. Secondary outcomes: Mini-AQLQ at 12 months, lung function, 6-minute-walk test, physical activity level, Nijmegen Questionnaire, Hospital Anxiety and Depression Scale, and adverse events. Repeated-measures mixed-effects models were used to analyze data. Poisson regression models were used to analyze adverse event incidence rate ratio.
Results
A total of 193 participants were allocated to UC + BrEX (n = 94) or UC (n = 99). UC + BrEX was superior in the primary outcome (adjusted mean change difference, 0.35; 95% confidence interval [CI], 0.07 to 0.62). Superiority in Mini-AQLQ was sustained at 12 months (0.38; 95% CI, 0.12 to 0.65). A minor improvement in Hospital Anxiety and Depression Scale depression score at 6 months favoring UC + BrEX (−0.90; 95% CI, −1.67 to −0.14) was observed. Asthma-related adverse events occurred similarly in UC + BrEX and UC participants: 14.9% versus 18.1% (P = 0.38).
Conclusions
BrEX as add-on to usual care improve asthma-related QoL in incompletely controlled asthma regardless of severity and with no evidence of harm.
Clinical trial registered with www.clinicaltrials.gov (NCT 03127059).
Keywords: asthma, quality of life, breathing exercises
Asthma is a common chronic and heterogeneous disease characterized by reversible airway obstruction, airway inflammation, and bronchial hyperresponsiveness causing dyspnea, wheezing, chest tightness, coughing, and impaired quality of life (QoL) for most patients (1, 2).
In addition to appropriate pharmacotherapy, self-management education, correct inhaled technique, adherence to recommended treatment, and treatment of relevant comorbidities, are key aspects of achieving asthma control (1, 3). Asthma severity is defined by the degree of pharmacological treatment required to achieve control and may be categorized by the daily dose of inhaled corticosteroids (ICS) and the requirement for second controllers, expressed as treatment steps 1–5 according to Global Initiative for Asthma (GINA) (1, 4). Most patients requiring high amounts of pharmacotherapy should be under specialist care (1). Drug and device effectiveness has improved markedly, but complete asthma control, defined by the absence of symptoms and exacerbations (1, 3), is achieved by less than 50% of patients with moderate to severe asthma (1, 5, 6), and aggressive pharmacologic escalation strategies fail to prevent persisting symptoms and QoL impairment for some (7), suggesting that additional nonpharmacological interventions may be helpful.
Breathing pattern abnormalities have been described in asthma (8), and randomized clinical trials showed that interventions with breathing exercises (BrEX, sometimes called breathing retraining) aiming to normalize breathing pattern improved asthma-related QoL in mild to moderate asthma treated in primary care (8–10). BrEX intervention delivered by trained physiotherapists is a safe and relatively inexpensive intervention that has been investigated in a few randomized trials (8, 10–12). BrEX include breathing retraining, relaxation techniques, and practice of the methods in activity (13).
On the basis of this evidence, BrEX is now recommended as add-on treatment in mild to moderate asthma in GINA (1) and British Thoracic Society and The Scottish Intercollegiate Guidelines Network (BTS-SIGN) UK guidelines (14). However, the effects of BrEX in a more severe asthma population attending specialist clinics has not previously been rigorously investigated. A recent meta-analysis emphasized that no trials have investigated patients with moderate to severe asthma attending specialist asthma clinics adequately to allow an evidence-based recommendation (15).
We hypothesized that usual care with add-on BrEX in adults with incompletely controlled moderate to severe asthma managed in specialist asthma clinics would be superior in improving asthma-related QoL at 6 months compared with usual care alone.
Some of the results of this trial have previously been reported in a conference abstract (16).
Methods
Study Design, Participants, and Randomization
We report a two-armed parallel group assessor-blinded multicenter randomized controlled trial (RCT) conducted in Denmark at seven public respiratory hospital clinics (Naestved, Roskilde, Copenhagen (Hvidovre, Bispebjerg), Aalborg, Silkeborg, Odense) and one private clinic (Elsinore) covering both rural, semirural and urban communities.
The protocol was published (13), approved by Region Zealand Research Ethics Committee (SJ-552), and registered (NCT 03127059) before commencement. The trial complied with the Helsinki declaration and follows the Consolidated Standards of Reporting Trials statement (17). A pilot study was completed at Naestved Hospital November 2015 to March 2016 (18).
Full description of trial methods is available in the eMethods section of the data supplement.
Inclusion criteria were aged ⩾18 years, attending specialist care (more than two visits) with incomplete asthma control (Asthma Control Questionnaire [ACQ6] score of 1.5 or higher; changed to 0.8 or higher in January 2018 owing to low recruitment rates) (19). Exclusion criteria were pregnancy, previous BrEX training before inclusion, or severe comorbidity (i.e., patients with short life expectancy due to terminal illness, or severe physical impairments or mental disease that would prevent participation in the intervention as evaluated by the pulmonologist). The responsible pulmonologist screened for eligibility. In the Danish healthcare sector, mild to moderate asthma is treated in general practice and more severe asthma in specialist care; however, asthma severity was not evaluated at trial inclusion. Participants were allocated 1:1 by random number generation (EasyTrial Inc.) to either add-on BrEX (UC + BrEX) or usual care (UC). Randomization was concealed to outcome assessors and data analysts. Blinded interpretation (20) was published before unblinding (21).
Procedures
UC was delivered at all sites (planned or acute visits) as, for example, assessment of asthma control, pharmacotherapy adjustment, or self-care instructions (1).
BrEX were delivered at the seven hospital sites’ physiotherapy units by trained physiotherapists. BrEX were provided face to face at three individual sessions (60 + 30 + 30 min, 3–4 weeks apart) and included instructions for home practice (10 min twice daily), and a booklet, which describes the program and specific exercises thoroughly (E1 in the data supplement; the material can be reused if this publication and the first author are cited). BrEX complied with the program used in the large mild to moderate asthma RCT (10) and included, in brief, nasal inhalation; breathing from diaphragm and lower chest; normalization of the tidal volume; shoulder, neck, tongue, and jaw relaxation; exhalation to functional residual capacity; aiming for respiration frequency of 12–16/min; suppression techniques (if frequent yawns, dry coughs, or sighs); exhalation prolongation and/or breath-hold technique (if elevated respiration frequency) and starting at relaxed body position progressing to use during physical activity. The trial protocol describes BrEX in detail (13).
Outcomes
Primary outcome
Between-group difference in 6-month mean Mini-AQLQ change (intention-to-treat) (9). Mini-AQLQ is a simple-to-administer and well-validated 15-item patient-reported questionnaire using a seven-point Likert scale (1 = maximally impaired; 7 = not impaired).
Secondary outcomes
Patient-reported outcome measures (PROMs): Mini-AQLQ (at 3 and 12 mo), asthma symptoms were assessed by six-item Asthma Control Questionnaire (ACQ6) (22), dysfunctional breathing–related symptoms by Nijmegen Questionnaire (NQ, cutoff ⩾ 23 point) (23), psychological domains by Hospital Anxiety and Depression Scale (HADS) (24); adverse events (AE), and baseline demography.
Objective physiological assessments: 6-minute-walk test (6MWT) was performed to assess functional exercise capacity. To assess lung function, percentage of predicted forced expiratory volume in the first second (FEV1%pred) was used (MedikroPro spirometer; Medikro Oy). Reference values: Global Lung Functions Initiative 2012 (25). Accelerometry (BodyMedia SenseWear): average steps per day and physical activity level (PAL) during a 6-day period (23 h/d) (26) were included to learn whether a possible change in asthma-related QoL would translate into increased PAL as a surrogate marker of less limitation in lived everyday life.
Extracted from medical records
Comorbidities to describe the participants; acute or planned hospital or emergency care visits to monitor AEs; prescribed asthma medication to define asthma severity classification according to GINA treatment steps (1).
Outcomes are presented in detail in protocol (13). Details on GINA step classification, AE questionnaire, AE classification, BrEX session attendance, and home exercise adherence are provided in Sections E10–E12 in the data supplement.
Statistical Analysis
The statistical analysis plan was available online before trial completion (27).
Sample size calculation was based on a mean effect size of 0.38 in Mini-AQLQ (12), standard deviation twice the effect size (28), 90% power, and P value (two-sided) of 0.05, requiring 172 participants (86 in each group). We expected 10% attrition and aimed to enroll 190 participants.
Repeated-measures mixed-effects model with subject being a random factor and treatment arm, visit (i.e., baseline, 3-month, 6-month, and 12-month follow-up), and interaction between treatment arm and visit as fixed factors, with adjustment for treatment center, were used in the primary outcome analysis. Similar methods were used for the secondary outcomes. Per-protocol analyses were also performed using mixed-effects model including UC + BrEX participants attending three BrEX sessions and all UC participants. Post hoc, the primary outcome analysis was adjusted for NQ score (cutoff ⩾ 23) in a sensitivity analysis (27). Numbers needed to treat calculated based on the proportions of patients in each group improving or deteriorating from baseline to 6 months by the minimum important difference (MID) of 0.5 in the Mini-AQLQ score (29). Post hoc analysis of odds of MID from BrEX at 6 months was performed. AE incidence rate ratio comparisons between groups were reported using Poisson regression models with treatment center, GINA step, and body mass index as covariates.
We used STATA 16.1 (StataCorp) for analyses.
Results
Between April 2017 and September 2019, 314 patients were screened for eligibility, and 193 were randomized, 94 to UC + BrEX and 99 to UC (Figure 1, Table E2A, and Figure E2B). Baseline characteristics were similar between groups (Table 1; comorbidity data: see Table E3).
Figure 1.
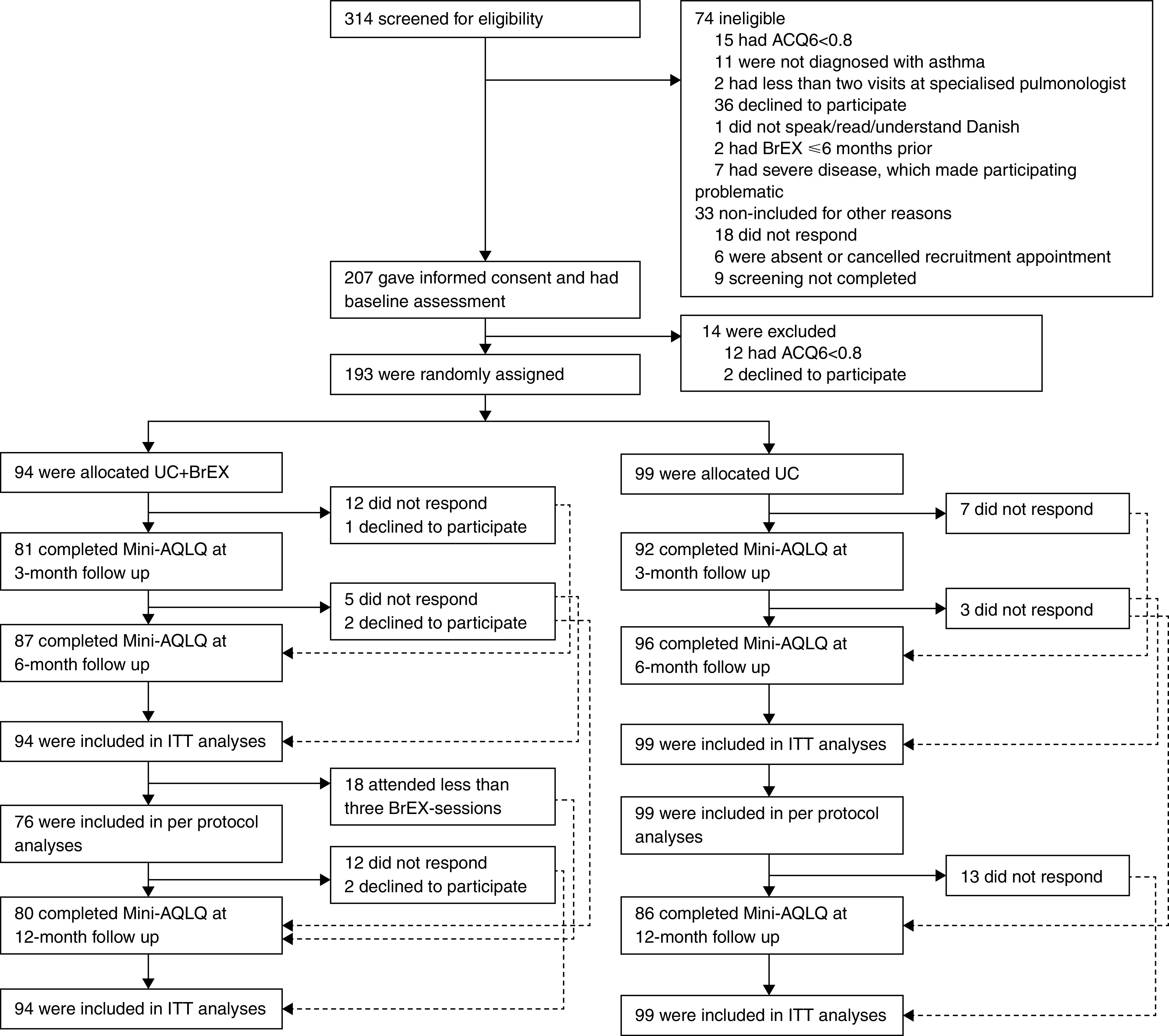
Trial profile. ACQ6 = 6-Item Asthma Control Questionnaire; BrEX = breathing exercises; ITT = intention-to-treat; Mini-AQLQ = Mini-Asthma Quality of Life Questionnaire; UC = usual care alone.
Table 1.
Baseline characteristics
| UC + BrEX (n = 94) | UC (n = 99) | |||||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | — | 58 | (61.7%) | — | 64 | (64.7%) |
| Male | — | 36 | (38.3%) | — | 35 | (35.4%) |
| Age at inclusion | — | 55 | (44–65) | — | 51 | (42–61) |
| Smoking status | ||||||
| Never-smokers | — | 89 | (55.3%) | — | 95 | (65.7%) |
| Smokers | — | 5 | (5.3%) | — | 4 | (4.0%) |
| Former smokers | — | 37 | (39.4%) | — | 30 | (30.3%) |
| Body mass index | ||||||
| Underweight | — | 1 | (1.1%) | — | 1 | (1.0%) |
| Normal weight | — | 24 | (25.5%) | — | 22 | (22.2%) |
| Overweight | — | 29 | (30.9%) | — | 44 | (44.4%) |
| Obese | — | 26 | (27.7%) | — | 20 | (20.2%) |
| Severely obese | — | 8 | (8.5%) | — | 7 | (7.1%) |
| Extremely obese | — | 6 | (6.4%) | — | 5 | (5.1%) |
| PROMs | ||||||
| Mini-AQLQ | — | 4.3 | (3.7–5.1) | — | 4.4 | (3.6–5.1) |
| ACQ6 | — | 2.2 | (1.5–2.7) | — | 2.0 | (1.2–2.7) |
| NQ* | — | 22.9 | (10.9) | — | 23.1 | (11.3) |
| HADS-anxiety | — | 5 | (3–10) | — | 6 | (3–9) |
| HADS-depression | — | 3 | (1–7) | — | 3 | (1–6) |
| EQ-5D-5L index | — | 0.742 | (0.648–0.859) | — | 0.754 | (0.700–0.824) |
| EQ-5D-5L VAS* | — | 62.0 | (20.7) | — | 62.1 | (19.0) |
| GINA steps | ||||||
| 1 | — | 0 | (0%) | — | 0 | (0%) |
| 2 | — | 1 | (1.1%) | — | 2 | (2.0%) |
| 3 | — | 16 | (17.0%) | — | 13 | (13.1%) |
| 4 | — | 31 | (33.0%) | — | 34 | (34.3%) |
| 5 | — | 46 | (48.9%) | — | 50 | (50.5%) |
| Inhaled corticosteroids | ||||||
| None | — | 1 | (1.1%) | — | 1 | (1.0%) |
| Low | — | 18 | (19.2%) | — | 20 | (20.2%) |
| Moderate | — | 33 | (35.1%) | — | 30 | (30.3%) |
| High | — | 42 | (44.7%) | — | 48 | (48.5%) |
| Number of second controller(s) | ||||||
| None | — | 5 | (5.3%) | — | 4 | (4.0%) |
| 1 | — | 41 | (43.6%) | — | 45 | (45.5%) |
| 2 | — | 30 | (31.9%) | — | 35 | (35.4%) |
| 3 | — | 14 | (14.9%) | — | 13 | (13.1%) |
| 4+ | — | 4 | (4.3%) | — | 2 | (2.0%) |
| Oral corticosteroids† | — | 6 | (6.4%) | — | 2 | (2.0%) |
| Biological treatment | — | 13 | (13.8%) | — | 9 | (9.1%) |
| Objective measures | ||||||
| 6-min-walk test (m) | (n = 90) | 467 | (422–528) | (n = 97) | 469 | (417–515) |
| Borg CR10 (resting) | (n = 94) | 1 | (0.3–2.5) | (n = 99) | 2 | (0.5–2.5) |
| FEV1% predicted | (n = 85) | 80 | (73–87) | (n = 91) | 80 | (66–90) |
| FEV1:FVC ratio | (n = 85) | 0.73 | (0.66–0.80) | (n = 91) | 0.73 | (0.67–0.79) |
| PEFR (L/min) | (n = 85) | 359 | (308–421) | (n = 91) | 355 | (282–434) |
| Steps per day (avg 6 d) | (n = 41) | 7046 | (4637–9517) | (n = 44) | 7278 | (4899–10175) |
| PAL (avg 6 d) | (n = 41) | 1.5 | (1.4–1.6) | (n = 44) | 1.5 | (1.4–1.6) |
Definition of abbreviations: ACQ6 = 6-item Asthma Control Questionnaire; Borg CR10= Borg Category-Ratio10; EQ-5D-5L = EuroQol (EQ-5D) instrument, 5 dimensions 5 levels generic quality of life; FEV1%predicted = predicted percentage of forced expiratory volume in the first second; FVC = forced vital capacity; GINA = Global Initiative for Asthma; HADS = Hospital Anxiety and Depression Scale; Mini-AQLQ = Mini-Asthma Quality of Life Questionnaire; NQ = Nijmegen Questionnaire; PAL = average physical activity level per day; PEFR = peak expiratory flow rate; PROMs = patient-reported outcome measures; UC = usual care alone; UC + BrEX = breathing exercises and usual care; VAS = visual analogue scale.
Data are reported as medians with interquartile range and frequency with percentage unless otherwise indicated.
Means and standard deviations.
Maintenance oral corticosteroids.
At 6 months’ follow-up, 183 (94.8%) participants completed Mini-AQLQ (Figure 1). For other outcomes, different inconsistencies in data collection were seen (Table E4). Follow-up assessment of physiological outcomes was limited by coronavirus disease (COVID-19)-related attendance issues. Accelerometry data were collected in 93 participants (i.e., those with data available for ⩾6 d during baseline assessment or 3- or 6-month follow-up).
A total of 76 (80.9%) UC + BrEX versus 99 (100%) UC subjects completed full protocol requirements and were included in the per-protocol analyses (Figure 1).
Primary Outcome
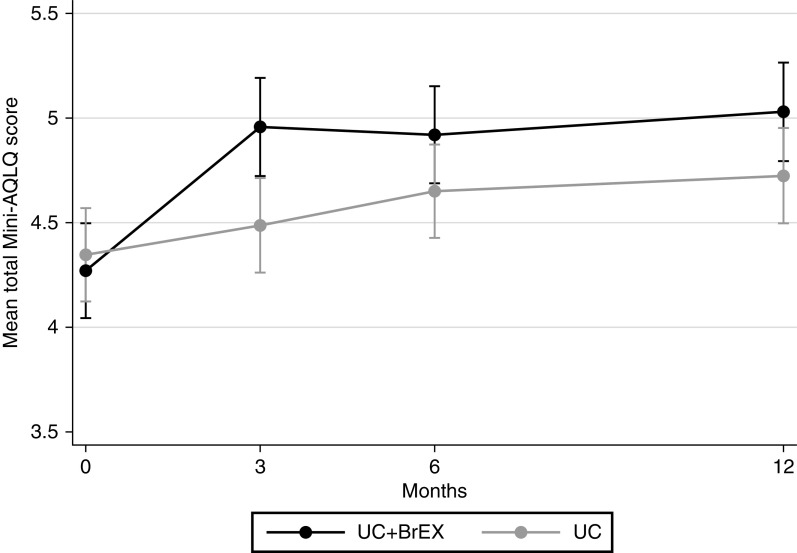
We observed a statistically significant adjusted difference favoring UC + BrEX of 0.35 Mini-AQLQ points (95% confidence interval [CI], 0.07–0.62) (Table 2 and Figure 2).
Table 2.
Adjusted intention-to-treat analyses and per-protocol analyses of Mini-Asthma Quality of Life Questionnaire and secondary outcomes at 6 months
| Total No. of Assessments* |
Intent-to-Treat Population |
|||||||
|---|---|---|---|---|---|---|---|---|
| UC + BrEX (n = 94) | UC (n = 99) | Between-Group Difference | ||||||
| UC + BrEX | UC | Mean Change | Mean Change | Difference in Mean Change | ||||
| Mini-AQLQ | 262 | 287 | 0.65 | (0.46 to 0.85) | 0.31 | (0.12 to 0.49) | 0.35 | (0.07 to 0.62) |
| ACQ6 | 256 | 285 | −0.32 | (−0.5 to −0.15) | −0.21 | (−0.38 to −0.05) | −0.11 | (−0.35 to 0.13) |
| NQ | 255 | 285 | −3.83 | (−5.52 to −2.13) | −2.78 | (−4.39 to −1.17) | −1.05 | (−3.38 to 1.29) |
| HADS-anxiety | 255 | 284 | −1.06 | (−1.73 to −0.38) | −1.11 | (−1.75 to −0.47) | 0.06 | (−0.87 to 0.98) |
| HADS-depression | 255 | 284 | −1.16 | (−1.71 to −0.61) | −0.26 | (−0.78 to 0.27) | −0.90 | (−1.67 to −0.14) |
| 6MWT (m) | 160 | 176 | 2.03 | (−10.2 to 14.27) | 9.03 | (−2.44 to 20.5) | −7.00 | (−23.77 to 9.77) |
| FEV1% pred | 150 | 163 | 0.48 | (−2.19 to 3.14) | −0.53 | (−3.01 to 1.96) | 1.00 | (−2.64 to 4.65) |
| Steps per day | 82 | 89 | 84.74 | (−973.24 to 1,142.72) | −245.85 | (−1282.1 to 790.4) | 330.59 | (−1149.86 to 1,811.04) |
| PAL | 82 | 89 | 0.03 | (−0.02 to 0.08) | −0.02 | (−0.06 to 0.03) | 0.05 | (−0.02 to 0.11) |
| Total No. of Assessments* |
Per-Protocol Population |
|||||||
|---|---|---|---|---|---|---|---|---|
| UC + BrEX (n = 76) | UC (n = 99) | Between-Group Difference | ||||||
| UC+BrEX | UC | Mean Change | Mean Change | Difference in Mean Change | ||||
| Mini-AQLQ | 222 | 287 | 0.68 | (0.47 to 0.89) | 0.31 | (0.12 to 0.49) | 0.38 | (0.1 to 0.66) |
| ACQ6 | 216 | 285 | −0.39 | (−0.58 to −0.2) | −0.21 | (−0.38 to −0.05) | −0.18 | (−0.43 to 0.07) |
| NQ | 215 | 285 | −4.03 | (−5.88 to −2.19) | −2.78 | (−4.41 to −1.16) | −1.25 | (−3.71 to 1.21) |
| HADS-anxiety | 215 | 284 | −1.13 | (−1.84 to −0.42) | −1.11 | (−1.74 to −0.48) | −0.02 | (−0.97 to 0.93) |
| HADS-depression | 215 | 284 | −1.46 | (−2.03 to −0.89) | −0.26 | (−0.76 to 0.25) | −1.20 | (−1.97 to −0.44) |
| 6MWT (m) | 140 | 176 | 2.50 | (−10.2 to 15.19) | 9.03 | (−2.53 to 20.58) | −6.53 | (−23.69 to 10.63) |
| FEV1% pred | 131 | 163 | 0.87 | (−1.89 to 3.63) | −0.52 | (−3.02 to 1.99) | 1.39 | (−2.34 to 5.11) |
| Steps per day | 79 | 89 | 139.09 | (−921.97 to 1,200.14) | −248.85 | (−1282.53 to 784.83) | 387.93 | (−1093.4 to 1,869.27) |
| PAL | 79 | 89 | 0.03 | (−0.02 to 0.08) | −0.01 | (−0.06 to 0.03) | 0.05 | (−0.02 to 0.11) |
Definition of abbreviations: 6MWT = 6-min-walk test; ACQ6 = 6-item Asthma Control Questionnaire; FEV1% pred = predicted percentage of forced expiratory volume in the first second; HADS = Hospital Anxiety and Depression Scale; Mini-AQLQ = Mini-Asthma Quality of Life Questionnaire; NQ = Nijmegen Questionnaire; PAL = average physical activity level per day; UC = usual care alone; UC + BrEX = breathing exercises and usual care.
Data are adjusted mean change from baseline to 6 months including 95% confidence interval.
Possible assessments for questionnaires (at baseline + at 3 mo + at 6 mo): 282 for UC + BrEX (in per-protocol: 228) and 297 for UC; for FEV1% pred and 6MWT (at baseline + at 6 mo): 188 for UC + BrEX (in per-protocol: 152) and 198 for UC; steps per day and PAL (at baseline + at 3 mo + at 6 mo): 135 for UC + BrEX (in per-protocol population: 126) and 144 for UC.
Figure 2.
Mean total Mini-Asthma Quality of Life Questionnaire (Mini-AQLQ). Comparison of groups showing asthma-related quality of life (Mini-AQLQ [95% confidence interval]) at baseline, 3-month, 6-month (primary outcome), and 12-month follow-up. Higher score denotes improved quality of life. UC = usual care alone; UC + BrEX = usual care and breathing exercises.
Secondary Outcomes: Mini-AQLQ
A difference favoring UC + BrEX was also seen at 3-month (0.56 [0.28–0.85]) and 12-month follow-up (0.38 [0.12–0.65]) (Figure 2 and Tables E5 and E6). The primary endpoint result was identical when adjusted for baseline NQ score (cutoff ⩾ 23 points; 0.34 [0.07–0.62]) (Table E7b), and in the per-protocol analysis (0.38 [0.10–0.66]) (Table 2).
Numbers needed to treat for one participant to improve at least 0.5 Mini-AQLQ units at 6 months by UC + BrEX was 7.6 (Table E8b). In UC + BrEX, a 6-month improvement of at least 0.5 in Mini-AQLQ was observed in 47 (54%) and deterioration in 11 (13%) versus 40 (42%) and 17 (18%), respectively, in UC (P = 0.24). Odds of a clinically meaningful outcome at 6 months were not significant, with an odds ratio (95% CI) of 1.48 (0.83–2.61). In within-group analyses, both study groups improved at 6-month follow-up: UC + BrEX, 0.65 (0.46–0.85), and UC, 0.31 (0.12–0.49).
Secondary Outcomes: Other
The only statistically significant 6-month between-group difference was a change in HADS-depression of −0.9 (−1.67 to −0.14) favoring UC + BrEX. Several numerical yet nonsignificant improvements at 6 months were seen in most PROMs favoring UC + BrEX (Table 2).
Within-group improvements in PROMs were observed in both groups for ACQ6, NQ, and HADS-anxiety, but not in any physiological outcomes (6MWT, FEV1%pred, steps per day, or daily PAL) (Table 2).
Global perceived effect improvements at 6 months were reported by 43.0% of UC + BrEX compared with 30.9% of UC subjects (P = 0.09) (Figure E9b and Table E9c).
GINA treatment steps were unchanged during the trial in the majority (UC + BrEX 84.0% vs. UC 82.7%) with similar proportions stepped up (6.4% vs. 5.1%) and stepped down (9.6% vs. 12.3%; P = 0.51) (Table E10a).
Asthma-related AEs causing unscheduled healthcare contact were observed in a total of 32 participants: 34 AEs in 14 participants in the UC + BrEX group versus 39 AEs in 18 participants in the UC group (P = 0.38). Asthma exacerbations needing oral corticosteroids occurred in 7.5% of UC + BrEX participants versus 5.1% UC participants (P = 0.70) (Table 3). Asthma-related severe adverse events (SAEs) with/without oral corticosteroid course occurred in 9.6% versus 10.2% of participants (P = 0.79), respectively (Table E11d).
Table 3.
Asthma-related adverse events, asthma-related serious adverse events, and courses of oral corticosteroids
| UC + BrEX |
UC |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of Participants | Number of Events | Number of Participants | Number of Events | IRR* | P Value | |||
| Adverse events | — | — | — | — | — | — | 1.47 | 0.381 |
| 0 | 80 | (85.1%) | 0 | 81 | (81.8%) | 0 | — | — |
| 1 | 11 | (11.7%) | 11 | 12 | (12.1%) | 12 | — | — |
| 2 | 1 | (1.1%) | 2 | 3 | (3.0%) | 6 | — | — |
| 3+ | 2 | (2.1%) | 21 | 3 | (3.0%) | 21 | — | — |
| Total | 94 | (100%) | 34 | 99 | (100%) | 39 | — | — |
| Serious adverse events | — | — | — | — | — | — | 2.03 | 0.159 |
| 0 | 88 | (93.6%) | 0 | 90 | (90.9%) | 0 | — | — |
| 1 | 6 | (6.4%) | 6 | 6 | (6.1%) | 6 | — | — |
| 2 | 0 | (0%) | 0 | 3 | (3.0%) | 6 | — | — |
| Deaths | 0 | (0%) | 0 | 0 | (0%) | 0 | — | — |
| Total | 94 | (100%) | 6 | 99 | (100%) | 12 | — | — |
| OCS courses† | — | — | — | — | — | — | 0.82 | 0.704 |
| 0 | 87 | (92.6%) | 0 | 93 | (94.9%) | 0 | — | — |
| 1 | 6 | (6.4%) | 6 | 3 | (3.1%) | 3 | — | — |
| 2 | 1 | (1.1%) | 2 | 2 | (2.0%) | 4 | — | — |
| Total | 94 | (100%) | 8 | 98 | (100%) | 7 | — | — |
Definition of abbreviations: IRR = incidence rate ratio; OCS = oral corticosteroids; UC = usual care alone; UC+BrEX = breathing exercises and usual care.
UC group compared with UC + BrEX group.
One missing in UC group.
A total of 505 all-type AEs were reported by 150 participants, 259 events (51.1%) in 73 UC + BrEX participants versus 246 events (48.9%) in 77 UC (P = 0.66) participants, and 14 SAEs were observed in 14 UC+BrEX participants versus 21 SAEs in 17 UC participants (P = 0.28), most commonly asthma exacerbations (Tables E11a and E11b).
There was no difference in incidence rates of all types of SAEs (P ⩾ 0.28) or asthma-related SAEs (P ⩾ 0.16) between groups (Table 3 and Table E11a). No SAEs were considered to be trial related.
All three physiotherapist-delivered sessions were attended by 76 (81%), with 1 (1%) attending none, 11 (12%) one, and 6 (6%) attending two sessions. Adherence to home exercises was estimated by physiotherapists as “good” or “excellent” (numeric rating scale [NRS] 4–5) in 76% (Section E12).
Discussion
In recent clinical guidelines, BrEX are advocated as add-on treatment to improve QoL for patients with mild to moderate asthma with persisting impaired control, but despite anecdotal reports of effectiveness in the difficult asthma setting, rigorous RCT evidence has until now been lacking (1, 14, 15). Our large multicenter RCT on poorly controlled moderate to severe asthma (in specialist care) showed that add-on BrEX delivered as three sessions by trained physiotherapists supplemented by daily home exercises are safe and effective in improving QoL at 6 months (primary endpoint) with sustained improvements after 12 months. The effect size was similar to that observed in studies in milder disease, and, likewise, without any measurable effect in physiological variables, including lung function. This is novel evidence that completes the circle: BrEX are now an evidence-based treatment option for the large cohort of patients, regardless of asthma severity, with impaired asthma control despite standard pharmacological management (2, 5–7).
The individual patient MID of Mini-AQLQ is 0.5 units, whereas the MID for between-group mean differences in controlled studies is unclarified (29, 30) and varies according to population and context (31). Meta-analysis on pharmacological placebo-controlled trials reports that a between-group difference of at least 0.5 is unachievable, and that smaller differences indicate clinically relevant benefits (6). Indeed, the interpretation advice of the AQLQ developers explicitly states that important benefits are associated with differences of less than 0.5 (29). In a large meta-analysis on AQLQ effect by asthma therapy, biological treatment had an effect size of 0.31 (95% CI, 0.20–0.41) and long-acting beta2-agonist (LABA) 0.35 (0.27–0.43) in patients uncontrolled on ICS alone, with smaller effect for other options (6). The validity of Mini-AQLQ and AQLQ is considered comparable (9). Thus, BrEX seem to confer an improvement equal to or greater than add-on controllers. The effect size in our trial was larger at both 6 and 12 months than that reported in milder asthma at 12 months (0.24 [95% CI, 0.04–0.44]) (10), and equal to that reported in a recent Cochrane review (15) of mild to moderate asthma (at 3 months, 0.42 [95% CI, 0.17–0.68]), including four different kinds of breathing interventions (Buteyko, Pranayama, yoga, and BrEX) and small studies (i.e., n = 57 participants). The odds for achieving MID at 6 months in our trial were equal to findings in milder asthma (odds ratio, 1.44 [95% CI, 0.88–2.36]) (15).
In recent years, relatively small studies (i.e., numbers randomized were less than 60, and thus 30 in the BrEX arm) have been published reporting variations of BrEX protocols, but more complex than our intervention: Pranayama breathing exercise compared with aerobics (32) or relaxation (33); add-on BrEX to speech therapy (34); and larger dose (i.e., 11–24 sessions) (32, 34). Two studies showed effects in AQLQ and asthma symptoms (33, 34), whereas aerobics and Pranayama showed equal effects in both AQLQ and ACQ6 (32). Besides the differences in design, the asthma severity due to degree of pharmacotherapy is not reported (32–34), thus hardly comparable to our trial.
Our results are comparable to those from exercise-based pulmonary rehabilitation (AQLQ improved 0.39 [0.02–0.76]), reported in a recent meta-analysis in 198 patients with severity-unspecified asthma (35).
Mini-AQLQ was chosen as primary outcome as previous research (in milder disease) indicated that BrEX do not affect asthma control but predominantly affect QoL, the outcome measure reflecting a patient’s experience of living with their disease (8, 10, 12, 15). This finding was replicated in our more severe population, as was the observation that effect size was not associated with baseline NQ scores (10). Thus, benefit of BrEX seems in both mild to moderate and moderate to severe asthma to be independent of baseline NQ of ⩾23 points, a cutoff used in many studies to define the presence of dysfunctional breathing (10, 36) (Table E7). Our trial supports a consistent improvement of BrEX across all included PROMs, but with no improvement in lung function, PAL, or 6MWT (10, 15, 37, 38). Thus, there is no evidence that BrEX change asthma pathophysiology, leaving the biomechanical mechanism and relative effectiveness of components of the intervention unclear. In addition, the persistence of benefits and need for booster BrEX require further investigation, although in keeping with previous studies, we found that the benefits were maintained at 12 months.
Limitations
Our trial has some limitations. We were unable to blind participants to the intervention, which may lead to unspecific, contextual effects in the active group who experienced increased attention from the trial physiotherapist. In previous BrEX trials, attempts were made to control for attention by allocating the control group to a similar professional contact (i.e., a nurse providing nonpersonalized asthma education) (11, 12), but a subsequent trial without such active control showed similar between-group differences (10), suggesting that the observed effect is delivered by the BrEX content. Slow recruitment forced us to change the inclusion criterion of asthma control from uncontrolled (ACQ6 ⩾ 1.5) to incompletely controlled (ACQ6 ⩾ 0.8), yet median ACQ6 was above 2.0, with the majority of participants having ACQ6 of higher than 1.5 (Table 1) despite at least two visits in a specialized care clinic and most participants adhering to GINA treatment steps 4 or 5. Despite this optimization, participants had persisting impaired asthma control and QoL (4). Mini-AQLQ and NQ were deliberately not used as inclusion criteria to avoid selection bias.
Overall, we succeeded in recruiting a previously unstudied population, randomizing 193 participants, with >80% treated at GINA treatment steps 4 or 5, and a 94.8% retention rate for the primary outcome. A high attendance rate for the BrEX sessions with the physiotherapists and high scores in adherence to home exercise indicated that the intervention was well tolerated, acceptable, and practiced by most. The included sites represented all Danish health regions, with a number of different physiotherapists delivering BrEX, all experienced at providing BrEX, and attempted to standardization delivery as much as possible, observing consistent effects across sites. The multicenter and multitherapist aspects as well as the inclusion of larger and smaller outpatient departments give confidence to external validity, and similarly, the constancy of observed effect size with previous studies in milder asthma supports validity.
All data collection and analyses were blinded, and we published our conclusion while allocation was still blinded (21), so within the pragmatic, real-world setting, we made efforts to minimize sources of bias.
Conclusions
We found that add-on physiotherapist-delivered BrEX improve asthma-related QoL at 6 months with sustained effects at 12 months, without evidence of harm, in patients with incompletely controlled moderate to severe asthma receiving standard asthma care by respiratory specialists.
Our results suggest that BrEX should be offered to all patients with asthma, regardless of asthma severity, who experience impaired asthma control despite optimization on pharmacotherapy and other factors.
Acknowledgments
Acknowledgment
The authors thank all patients and the involved clinical and scientific staff at the participating sites.
Footnotes
Supported by noncommercial funding resources: Naestved, Slagelse and Ringsted Hospitals’ Research Fund, Region Zealand Health Scientific Research Foundation, the Danish Foundation TrygFonden (ID: 117031), and the Association of Danish Physiotherapist’s Research Fund. The funding resources had no role in the design, execution, analyses, interpretation, manuscript, or submission of this trial.
Author Contributions: K.H.A., S.T.S., M.T., and U.B. (research group [i.e., “steering committee”]) contributed substantively to the concept and design of this trial. The chief investigator (K.H.A.) developed manuals (recruitment, assessment, and treatment) and written information, applied for grants, assigned for approvals, registered at ClinicalTrials.gov, introduced and supervised the recruitment, assessment, and treatment procedures to all involved nurses and physiotherapists, and led the data collection and verification. U.B., S.T.S., and M.T. gave feedback. S.T.S. specifically contributed to description of the statistical analyses. K.H.A. analyzed data and drafted the manuscript. K.H.A., S.T.S., C.S.U., C.P., K.S., J.B.-N., K.D.A., H.M., M.T., and U.B. provided intellectual feedback to the manuscript and approved the final version.
Data sharing: Deidentified participant data collected for the trial will be made available according to Danish legislation on reasonable request (until 5 years after trial closure) upon contact with principal investigator K.H.A.: khad@regionsjaelland.dk.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global strategy for asthma management and prevention. 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
- 2. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet . 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 3. Papaioannou AI, Kostikas K, Zervas E, Kolilekas L, Papiris S, Gaga M. Control of asthma in real life: still a valuable goal? Eur Respir Rev . 2015;24:361–369. doi: 10.1183/16000617.00001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor DR, Bateman ED, Boulet L-P, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. Eur Respir J . 2008;32:545–554. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 5. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J . 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 6. Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol . 2015;136:914–922. doi: 10.1016/j.jaci.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 7. Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJH, Pedersen SE. The correlation between asthma control and health status: the GOAL study. Eur Respir J . 2007;29:56–62. doi: 10.1183/09031936.00128505. [DOI] [PubMed] [Google Scholar]
- 8. Burgess J, Ekanayake B, Lowe A, Dunt D, Thien F, Dharmage SC. Systematic review of the effectiveness of breathing retraining in asthma management. Expert Rev Respir Med . 2011;5:789–807. doi: 10.1586/ers.11.69. [DOI] [PubMed] [Google Scholar]
- 9. Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J . 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 10. Bruton A, Lee A, Yardley L, Raftery J, Arden-Close E, Kirby S, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med . 2018;6:19–28. doi: 10.1016/S2213-2600(17)30474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas M, McKinley RK, Freeman E, Foy C, Prodger P, Price D. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax . 2003;58:110–115. doi: 10.1136/thorax.58.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax . 2009;64:55–61. doi: 10.1136/thx.2008.100867. [DOI] [PubMed] [Google Scholar]
- 13. Andreasson KH, Skou ST, Ulrik CS, Madsen H, Sidenius K, Jacobsen JS, et al. Protocol for a multicentre randomised controlled trial to investigate the effect on asthma-related quality of life from breathing retraining in patients with incomplete asthma control attending specialist care in Denmark. BMJ Open . 2019;9:e032984. doi: 10.1136/bmjopen-2019-032984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levy Ml, Thomas M, Small I, Pearce L, Pinnock H, Stephenson P. Summary of the 2008 BTS/SIGN British Guideline on the management of asthma. Prim Care Respir J . 2009;18 Suppl 1:S1–S16. doi: 10.3132/pcrj.2008.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santino TA, Chaves GS, Freitas DA, Fregonezi GA, Mendonça KM. Breathing exercises for adults with asthma. Cochrane Database Syst Rev . 2020;3:CD001277. doi: 10.1002/14651858.CD001277.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreasson KH, Skou ST, Ulrik CS, Madsen H, Sidenius K, Porsbjerg C, et al. Breathing exercises for specialist care asthma patients: a multicentre randomised trial. Eur Respir J . 2021;58:RCT2901. [Google Scholar]
- 17. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. Consolidated Standards of Reporting Trials Group CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol . 2010;63:e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Andreasson KH.Patients with incomplete asthma control – effectiveness of breathing exercises and associations with asthma-specific quality of life. https://pure-portal.regsj.dk/en/publications/effectiveness-of-breathing-exercises-and-associations-with-asthma
- 19.Andreasson KH.Breathing Exercises in Asthma Targeting Dysfunctional Breathing (BEAT_DB) 2020. https://clinicaltrials.gov/ct2/show/NCT03127059
- 20. Järvinen TLN, Sihvonen R, Bhandari M, Sprague S, Malmivaara A, Paavola M, et al. Blinded interpretation of study results can feasibly and effectively diminish interpretation bias. J Clin Epidemiol . 2014;67:769–772. doi: 10.1016/j.jclinepi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Bodtger U, Skou ST, Thomas M. In: Andreasson KH, editor. University of Southern Denmark; 2020. https://portal.findresearcher.sdu.dk/da/publications/blinded-review-of-the-primary-endpoint-results-from-the-beat-db-t [Google Scholar]
- 22. Juniper EF, Svensson K, Mörk A-C, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med . 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23. van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res . 1985;29:199–206. doi: 10.1016/0022-3999(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 24. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand . 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brazeau A-S, Karelis AD, Mignault D, Lacroix M-J, Prud’homme D, Rabasa-Lhoret R. Test-retest reliability of a portable monitor to assess energy expenditure. Appl Physiol Nutr Metab . 2011;36:339–343. doi: 10.1139/h11-016. [DOI] [PubMed] [Google Scholar]
- 27.Andreasson KH, Skou ST, Petersen I, Bodtger U.2020. https://portal.findresearcher.sdu.dk/da/publications/statistical-analysis-plan-breathing-exercises-in-asthma-targeting-2
- 28. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care . 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ . 1998;316:690–693. doi: 10.1136/bmj.316.7132.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ehrs P-O, Nokela M, Ställberg B, Hjemdahl P, Wikström Jonsson E. Brief questionnaires for patient-reported outcomes in asthma: validation and usefulness in a primary care setting. Chest . 2006;129:925–932. doi: 10.1378/chest.129.4.925. [DOI] [PubMed] [Google Scholar]
- 31. King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res . 2011;11:171–184. doi: 10.1586/erp.11.9. [DOI] [PubMed] [Google Scholar]
- 32. Evaristo KB, Mendes FAR, Saccomani MG, Cukier A, Carvalho-Pinto RM, Rodrigues MR, et al. Effects of aerobic training versus breathing exercises on asthma control: a randomized trial. J Allergy Clin Immunol Pract . 2020;8:2989–2996.e4. doi: 10.1016/j.jaip.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 33. Erdoğan Yüce G, Taşcı S. Effect of pranayama breathing technique on asthma control, pulmonary function, and quality of life: a single-blind, randomized, controlled trial. Complement Ther Clin Pract . 2020;38:101081. doi: 10.1016/j.ctcp.2019.101081. [DOI] [PubMed] [Google Scholar]
- 34. von Bonin D, Klein SD, Würker J, Streit E, Avianus O, Grah C, et al. Speech-guided breathing retraining in asthma: a randomised controlled crossover trial in real-life outpatient settings. Trials . 2018;19:333. doi: 10.1186/s13063-018-2727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Z, Wang J, Xie Y, Li J. Effects of exercise-based pulmonary rehabilitation on adults with asthma: a systematic review and meta-analysis. Respir Res . 2021;22:33. doi: 10.1186/s12931-021-01627-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedeh FB, Von Bülow A, Backer V, Bodtger U, Petersen US, Vest S, et al. The impact of dysfunctional breathing on the level of asthma control in difficult asthma. Respir Med . 2020;163:105894. doi: 10.1016/j.rmed.2020.105894. [DOI] [PubMed] [Google Scholar]
- 37. Löwhagen O, Bergqvist P. Physiotherapy in asthma using the new Lotorp method. Complement Ther Clin Pract . 2014;20:276–279. doi: 10.1016/j.ctcp.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 38. Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax . 2007;62:1039–1042. doi: 10.1136/thx.2006.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]