
In this month’s issue of AnnalsATS, Dr. Kim and colleagues (pp. 1561–1569) present research using the five-center PROSPR (Population-based Research to Optimize the Screening Process) cohort to examine lung cancer screening (LCS) follow-up for normal and abnormal exams stratified by program centralization and comparing Black to White patients (1). They demonstrate that among 5,142 individuals that received baseline LCS with initial negative findings, those that received their care at centralized programs were twice as likely to receive a subsequent annual screen compared with those that received their care at decentralized programs (76.1% vs. 34.8%). In addition, Black individuals were half as likely to receive annual follow-up LCS as White individuals (29.4% vs. 65.0%), but this disparity was only found in decentralized rather than centralized programs. Regarding individuals with positive baseline screens, their study did not find a difference in follow-up between centralized and decentralized programs, and there were no differences between Black and White patients. Overall, their work is the first to demonstrate that LCS program centralization may improve screening disparities in care among Black individuals.
This study is consistent with prior studies showing that Black individuals are less likely to receive an initial LCS, and when they do undergo LCS, they are about 30% less likely to receive the recommended follow-up (2–4). These are highly concerning findings as Black men have the highest lung cancer incidence and mortality from lung cancer of any demographic group (5), and Black populations may derive greater relative mortality benefits from screening (6). If current trajectories in LCS uptake and adherence persist among Black populations, LCS has the potential to exacerbate rather than improve existing disparities in screening and may also magnify disparities in lung cancer presentation stage and treatment seen in this population (7, 8). This study demonstrates that some programs are able to achieve equitable screening follow-up between Black and White patients, called “centralized programs”, but a better understanding of what this means will be essential to create tailored and feasible interventions that can be used by other LCS programs.
In general, in a centralized LCS program, patients are referred to a dedicated LCS program, and the program personnel (e.g., LCS coordinator) handle several of the key functions of LCS (e.g., verifying eligibility, conducting shared decision-making, ordering the LCS, notifying the patient of their results, and managing follow-up care). In decentralized programs, the ordering primary care provider is responsible for all the key functions and refers the patient to specialists. Consistent with this article, several others have shown that centralized programs improved adherence to annual follow-up after an initial LCS (9, 10). Current guidelines do not recommend one program type over the other; instead, it is recommended to choose the program type that best matches the resources available with the needs of the local community, and in real-world practice, many programs may operate as a hybrid model, incorporating some elements of both centralized and decentralized practices (11).
Even though centralized programs may attain better annual adherence and improve disparities among Black populations and potentially others who face health disparities, it is unclear what components of a centralized program underlie these benefits. This is an essential question, as the majority of screening currently takes place in decentralized settings, and in most healthcare systems, it is likely not feasible to fully centralize LCS. In addition to screening coordinators, centralized LCS programs often employ several other structures and processes that support LCS. For example, centralized screening programs may benefit from more knowledgeable practitioners, multidisciplinary tumor boards to guide the management of screen-detected findings, steering committees to optimize the implementation and maintenance of the LCS program, and LCS registries to systematically track results and follow-up. In addition, centralized programs may employ several processes that could improve screening among underserved populations, including clinical reminders to identify eligible patients and patients due for screening, providing educational materials, addressing barriers to accessing care such as insurance coverage and transportation, and even holistic systems to assist patients through the screening care continuum, known as patient navigation (12). These structures and care processes may all lead to improved patient screening outcomes (Figure 1).
Figure 1.
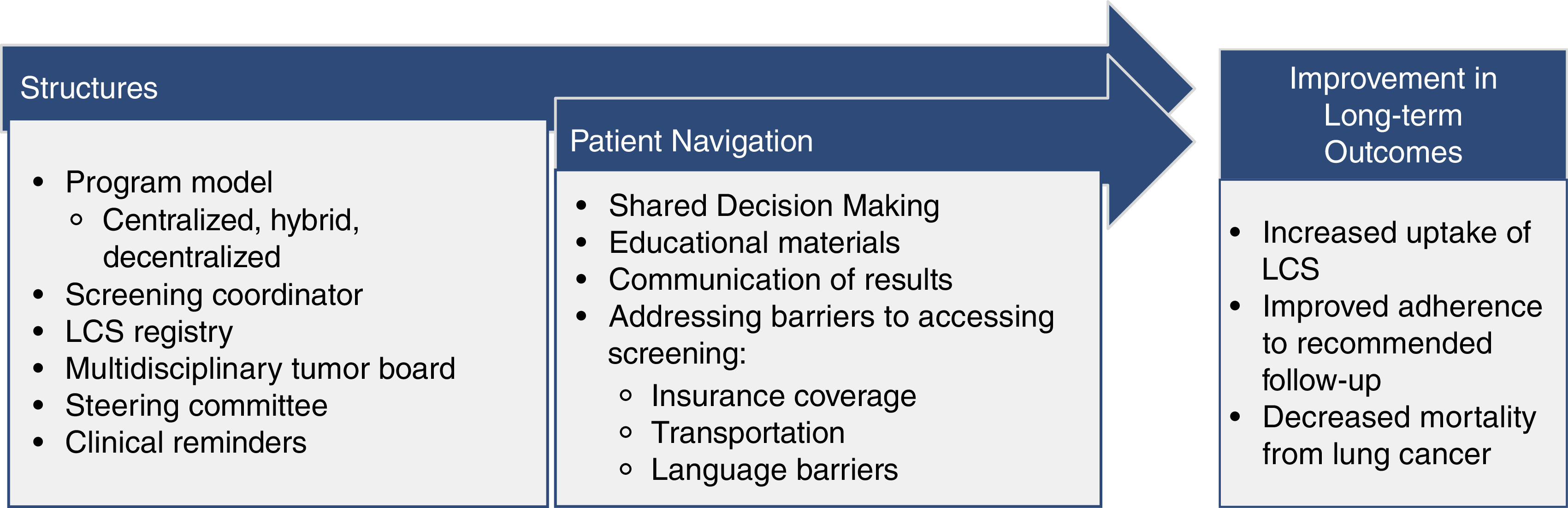
Potential structures and patient navigation processes in the lung cancer screening (LCS) continuum that improve disparities in screening among underserved populations.
We would argue that it may be the programmatic elements of patient navigation that can have the largest impact on mitigating disparities along the screening care continuum. Unmet social determinants of health (e.g., underinsurance, housing insecurity, and transportation) have been shown to be key reasons for having missed an LCS evaluation (13, 14). Patient navigation can generally be thought of as a set of patient-facing interventions to assist patients in overcoming barriers in a care process. These interventions have a strong evidence base in other screening modalities and have been demonstrated to improve screening uptake and adherence in limited studies in LCS (15). It is unclear to what extent these care processes were taking place at centralized sites in the current study from Dr. Kim and colleagues. A more in-depth analysis of the care processes which lead to improved screening follow-up in both this and other cohorts could be highly beneficial. In addition, prospective work incorporating key interventions to improve adherence that could be applied outside of centralized settings is needed, as navigation can often be used as a community-based rather than a program-based intervention.
There are also limitations to the conclusion that the centralization of programs is a solution to disparities in screening care. As the two centralized programs in this study were both health maintenance organizations, we do have some concern that disparities were partially mitigated by the higher socioeconomic status and adequate insurance coverage among members of these programs, though some of these factors were analyzed in adjusted analyses. Moreover, in only looking at the White and Black races, it is unclear that centralization improves care disparities in other populations. Centralization also likely does not address other disparities along the LCS care continuum, particularly in access to and uptake of screening.
Overall, this and other studies demonstrate that centralizing some features of an LCS program may improve screening care outcomes and equity. Future studies are needed to more clearly determine which structures and processes employed by these programs have the largest impact on improving LCS disparities among underserved populations and could be translated outside of a centralized setting. This work is critical as it will inform the implementation of future LCS strategies among diverse communities and healthcare systems with limited resources.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Kim RY, Rendle KA, Mitra N, Saia CA, Neslund-Dudas C, Greenlee RT, et al. Racial disparities in adherence to annual lung cancer screening and recommended follow-up care: a multicenter cohort study. Ann Am Thorac Soc . 2022;19:1561–1569. doi: 10.1513/AnnalsATS.202111-1253OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rustagi AS, Byers AL, Keyhani S. Likelihood of lung cancer screening by poor health status and race and ethnicity in US adults, 2017 to 2020. JAMA Netw Open . 2022;5:e225318. doi: 10.1001/jamanetworkopen.2022.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunitomo Y, Bade B, Gunderson CG, Akgün KM, Brackett A, Cain H, et al. Racial differences in adherence to lung cancer screening follow-up: a systematic review and meta-analysis. Chest . 2022;161:266–275. doi: 10.1016/j.chest.2021.07.2172. [DOI] [PubMed] [Google Scholar]
- 4. Núñez ER, Caverly TJ, Zhang S, Glickman ME, Qian SX, Boudreau JH, et al. Adherence to follow-up testing recommendations in US veterans screened for lung cancer, 2015–2019. JAMA Netw Open . 2021;4:e2116233. doi: 10.1001/jamanetworkopen.2021.16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin . 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 6. Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the national lung screening trial. Implications for widespread implementation. Am J Respir Crit Care Med . 2015;192:200–208. doi: 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 7. Potter AL, Rosenstein AL, Kiang MV, Shah SA, Gaissert HA, Chang DC, et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. BMJ . 2022;376:e069008. doi: 10.1136/bmj-2021-069008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Check DK, Albers KB, Uppal KM, Suga JM, Adams AS, Habel LA, et al. Examining the role of access to care: Racial/ethnic differences in receipt of resection for early-stage non-small cell lung cancer among integrated system members and non-members. Lung Cancer . 2018;125:51–56. doi: 10.1016/j.lungcan.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith HB, Ward R, Frazier C, Angotti J, Tanner NT. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest . 2022;161:818–825. doi: 10.1016/j.chest.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 10. Sakoda LC, Rivera MP, Zhang J, Perera P, Laurent CA, Durham D, et al. Patterns and factors associated with adherence to lung cancer screening in diverse practice settings. JAMA Netw Open . 2021;4:e218559. doi: 10.1001/jamanetworkopen.2021.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazzone PJ, Silvestri GA, Souter LH, Caverly TJ, Kanne JP, Katki HA, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest . 2021;160:e427–e494. doi: 10.1016/j.chest.2021.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin . 2011;61:237–249. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin D, Fishman MDC, Ngo M, Wang J, LeBedis CA. The impact of social determinants of health on lung cancer screening utilization. J Am Coll Radiol . 2022;19:122–130. doi: 10.1016/j.jacr.2021.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman A, Kross E, Crothers K, Cole A, Wernli K, Triplette M.Patient perspectives on longitudinal adherence to lung cancer screening Chest [online ahead of print] 8 Feb 2022; DOI: 10.1016/j.chest.2022.01.054 [DOI] [PubMed] [Google Scholar]
- 15. Shusted CS, Barta JA, Lake M, Brawer R, Ruane B, Giamboy TE, et al. The case for patient navigation in lung cancer screening in vulnerable populations: a systematic review. Popul Health Manag . 2019;22:347–361. doi: 10.1089/pop.2018.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]


