Abstract
Lung endothelial permeability is a key pathological feature of acute respiratory distress syndrome. Hyaluronic acid (HA), a major component of the glycocalyx layer on the endothelium, is generated by HA synthase (HAS) during inflammation and injury and is critical for repair. We hypothesized that administration of exogenous high molecular weight (HMW) HA would restore protein permeability across human lung microvascular endothelial cells (HLMVEC) injured by an inflammatory insult via upregulation of HAS by binding to CD44. A transwell coculture system was used to study the effects of HA on protein permeability across HLMVEC injured by cytomix, a mixture of IL‐1β, TNFα, and IFNγ, with or without HMW or low molecular weight (LMW) HA. Coincubation with HMW HA, but not LMW HA, improved protein permeability following injury at 24 h. Fluorescence microscopy demonstrated that exogenous HMW HA partially prevented the increase in “actin stress fiber” formation. HMW HA also increased the synthesis of HAS2 mRNA expression and intracellular HMW HA levels in HLMVEC following injury. Pretreatment with an anti‐CD44 antibody or 4‐methylumbelliferone, a HAS inhibitor, blocked the therapeutic effects. In conclusion, exogenous HMW HA restored protein permeability across HLMVEC injured by an inflammatory insult in part through upregulation of HAS2.
Keywords: acute lung injury, CD44, glycocalyx, hyaluronan, hyaluronic acid synthase, protein permeability
Abbreviations
- AKT
protein kinase B
- ALI
acute lung injury
- ARDS
Acute respiratory distress syndrome
- CD44
cluster of differentiation 44
- HA
hyaluronic acid
- HAS
hyaluronic acid synthase
- HAS2‐AS1
HAS2‐Antisense RNA 1
- HLMVEC
human lung microvascular endothelial cells
- HMGA2
high‐mobility Group AT‐hook 2
- HMW
high molecular weight
- LMW
low molecular weight
- 4‐MU
4‐Methylumbelliferone
- PI3K
phosphatidylinositol‐3‐kinase
- TLR
toll‐like receptor
- VE‐Cadherin
vascular endothelial‐cadherin
- ZO‐1
zonula occludens‐1
1. INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in critically ill patients in the intensive care units. 1 , 2 It is characterized by diffuse inflammatory injury to the lung capillary endothelium and alveolar epithelium, leading to the development of pulmonary edema formation. Despite improvements in the management of ARDS, mortality rates remain high, potentially up to 40%. In addition, there are no pharmacological therapies that improve survival. 3 Therefore, new therapeutic strategies for ARDS are needed.
In our previous study, administration of high molecular weight hyaluronic acid (HMW HA) suppressed inflammation and decreased protein permeability, pulmonary edema formation, and bacterial growth in an ex vivo perfused human lung injured with severe Escherichia coli bacterial pneumonia. 4 However, the mechanisms underlying the therapeutic effects remained largely unknown. HA or hyaluronan is a nonsulfated glycosaminoglycan, composed of repeating polymeric disaccharides d‐glucuronic acid and N‐acetyl‐d‐glucosamine, and a major component of both the extracellular matrix and endothelial surface glycocalyx layer, critical for homeostasis in the alveolar air–blood barrier and endothelial barrier integrity. 5 , 6 , 7 , 8 Under physiologic conditions, HA exists primarily as a HMW form (>1 MDa). During acute lung injury (ALI), HA is degraded by hyaluronidases, reactive oxygen and nitrogen species, and other inflammatory mediators into low molecular weight HA (LMW HA <500 kDa). 9 Surprisingly, based on its MW, HMW and LMW HA have opposing biological effects; LMW HA can decrease endothelial cell barrier function, stimulate angiogenesis, and induce inflammation, whereas HMW HA can exert anti‐inflammatory and immunosuppressive effects. 10 , 11 , 12 , 13 , 14 , 15 HA influences cell behavior through binding to various cell surface receptors such as CD44, TLR2 and 4, HABP2, or RHAMM. 16
HA is synthesized by HA synthase (HAS) 1–3, and HMW HA is a critical component of the glycocalyx layer 17 ; HAS1 and HAS2 produce HMW HA (500 kDa–2 MDa), whereas HAS3 produces LMW HA (<500 kDa). During injury, inflammatory cytokines can bind CD44 and increase the synthesis of intracellular HMW HA via stimulation of HAS2 in the endothelium, possibly as a reparative mechanism to restore the glycocalyx layer. 18 , 19 Similarly, exogenous HMW HA, which binds CD44, has been studied in various injury models as a mechanism to restore barrier integrity 20 ; Singleton et al. found that intravenous administration of HMW HA 4 h following LPS‐induced ALI improved endothelial permeability 21 via CD44, sphingosine 1 phosphate, and Akt and Rac signaling. 22 In this current study, we hypothesized that exogenous administration of HMW HA would restore protein permeability across human lung microvascular endothelial cells (HLMVEC) injured by an inflammatory insult via activation of CD44 and HAS2.
2. MATERIALS AND METHODS
2.1. Primary cultures of HLMVEC
HLMVEC (Lonza) were grown in a microvascular endothelial growth medium with 5% FBS and antibiotics and incubated in a humidified incubator with 5% CO2 at 37°C. The cells were used at a passage between 4 and 6. For permeability assays only, a transwell permeable support system (0.4 μm pore size containing a PTFE membrane that had been treated with an equimolar mixture of types I and III bovine placental collagen) that could promote cell attachment and spreading was used (#3495, Costar) based on our previous publication. 23 HLMVEC were grown on the insert at a density of 1 × 106 cells/well and maintained in a humidified incubator with 5% CO2 at 37°C for at least 24 h to obtain an intact monolayer. Cytomix, a mixture of human IL‐1β, TNFα, and IFNγ (the final concentration was 50 ng/ml for each cytokine in the medium), which we previously used as a surrogate for acute respiratory distress pulmonary edema fluid, 23 , 24 , 25 was then added to the HLMVEC with or without HMW HA (1 MDa) or LMW HA (40 kDa) 100 μg/mL (LifeCore Biomedical). After 24 h, the culture medium in the inserts were aspirated and replaced with a culture medium containing FITC‐dextran (100 mg/ml, 70 kDa, Sigma Aldrich) for another 2 h. The unidirectional flux of FITC‐dextran from the upper chamber to the lower chamber was measured to calculate protein permeability. In separate experiments, HLMVEC were incubated with either an anti‐CD44‐blocking antibody (IM7, 1 μg/ml, BD Biosciences, CA, USA), anti‐TLR4 antibody (10 μg/ml, #AF1478, R&D Systems), or negative control IgG antibody. The concentration of anti‐CD44‐blocking antibody was based on our previous studies. 23 , 24 The concentration of the anti‐TLR4 antibody was based on the manufacturer’s recommendation. In additional experiments, 4‐methylumbelliferone (4‐MU) (Sigma‐Aldrich), a pan HAS inhibitor, was added simultaneously with the cytomix ±HA to suppress HAS mRNA expression. LDH was measured according to the manufacturer’s protocol (Roche). The concentration of 4‐MU was based on previous publications. 26 , 27
2.2. Fluorescence microscopy of tight and adherens junctions
HLMVEC (1 × 105 cells) were seeded onto Lab‐Tek II chamber slides (Nalge Nunc International) and grown until 90% confluency was obtained as previously described. 25 Twenty‐four hours after treatment with cytomix with or without HMW or LMW HA, the cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. After additional washing, the cells were permeabilized by 0.1% Triton X‐100 and then blocked with 1% BSA at room temperature. For F‐actin staining, the slides were incubated with FITC‐phalloidin (Thermo Fischer Scientific) for 30 min at 37°C. For ZO‐1 staining, slides were incubated with Alexa ZO‐1 antibodies (5 μg/ml, Thermo Fischer Scientific) for 1 h at room temperature. For VE‐cadherin staining, primary antibodies to VE‐cadherin (1:100, Cell Signaling) were used. After multiple washing with PBS, slides were then incubated with the secondary antibody Alexa Fluor 488‐conjugated Goat Anti‐Rabbit IgG (H1L) DS Grade (1:50, Invitrogen, Thermo Fischer Scientific) for 1 h at room temperature. Slides were then mounted with Vectashield with DAPI mounting medium (Vector Laboratories). Images were obtained by AxioVision SE64 (Carl Zeiss Microscopy, LLC).
2.3. Quantitative real‐time polymerase chain reaction
For mRNA expression, 1 × 106 HLMVEC were seeded onto 24 well‐plate and injured with cytomix with or without HMW or LMW HA. Four, 12, and 24 h after injury and treatment, the cells were collected by using RNeasy Mini Kit (QIAGEN Sciences). The RNA isolation technique was performed according to the protocol provided by Qiagen (www.qiagen.com) and as previously described. 25 The quality of the RNA was assessed with the NanoDrop ND‐1000 UV–Vis Spectrophotometer (NanoDrop Technologies); 260/280 nm absorbance ratios of 2.0–2.2 and 260/230 nm absorbance ratios of 1.8–2.2 indicated a pure RNA sample. The samples were quickly stored at −80°C for the next steps. Primers including the probes used for quantitative real‐time polymerase chain reaction (qRT‐PCR) were purchased from Life Technologies (Thermo Scientific). Human HAS1 (Hs00758053_m1), HAS2 (Hs00193435_m1), HAS3 (Hs00193436_m1), high‐mobility group AT‐hook 2 (HMGA2; Hs04397751_m1), HAS2 antisense RNA 1 (HAS2‐AS1;Hs03309447_m1), and Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH; Hs03929097_g1) were used for our study. High‐capacity RNA‐to‐cDNA Kit and TaqMan Fast Universal PCR Master Mix from Applied Biosystems were used for the qRT‐PCR assays (Thermo Scientific). These assays were conducted following the Two‐Step qRT‐PCR protocol described by Applied Biosystems. QPCR was performed by StepOnePlus Real‐Time PCR System (Thermo Scientific). The relative gene expression was analyzed by the comparative cycle threshold (Ct) method also referred to as the 2‐ΔΔCt method. The endogenous control GAPDH was used to calculate ΔCt values for the gene expression. All primers used were MIQE guideline compliant.
2.4. Western blot analyses
HLMVEC were injured with cytomix with or without HMW or LMW HA. After 24 h, the cells were lysed with NP40 cell lysis buffer (Thermo Scientific) containing protease inhibitors and phosphatase inhibitors (RPI) as previously described. 25 The protein concentration was measured using Pierce BCA assay kit (Thermo Fischer Scientific). Each protein was diluted to 500 μg/ml for the next steps. The protein samples were heated at 70°C for 10 min with Invitrogen NuPAGE LDS Sample Buffer and loaded onto Invitrogen NuPAGE 4%–12% Bis‐Tris Gel or Invitrogen NuPAGE 3%–8% Bis‐Tris Gel (Thermo Fischer Scientific). The gels were transferred onto PVDF membrane by iBlot 2 Gel Transfer Device (Thermo Fischer Scientific). The membrane was blocked with Pierce blocking buffer (Thermo Fischer Scientific) or washed buffer with 5% bovine serum albumin (BSA) (TBS Tween 20 Buffer [TBST]) for 1 h at room temperature on a shaker. After washing, the membrane was incubated with the primary antibody which was diluted with TBST with 5% BSA overnight on a shaker at 4°C. The membrane was washed with TBST and reacted with a secondary antibody which was diluted with TBST with 5% BSA for 1 h at room temperature on a shaker. The detection of the target protein was examined by Pierce ECL Western Blotting Substrate or SuperSignal West Dura Extended Duration Substrate (Thermo Fischer Scientific). Images were obtained by ChemiDoc MP Imaging System (Bio‐Rad) according to the manufacturer’s instructions. The chemiluminescent intensity was analyzed by densitometry (ImageJ software, NIH Image). For Western blots of caspase‐3, the blots were cut prior to the hybridization with the antibodies due to the significantly higher intensity of the pro‐caspase 3 band compared with the activated form.
For the primary antibodies, rat anti‐human/mouse CD44 (#14‐0441‐85, Thermo Fischer Scientific), rabbit anti‐human ZO‐1 (#5406, Cell Signaling Technology), rabbit anti‐human VE‐cadherin (#2158, Cell Signaling Technology), rabbit anti‐human HMGA2 (#5269, Cell Signaling Technology), mouse anti‐human pAKT (#MAB887, R&D Systems), mouse anti‐human AKT (# MAB2055, R&D Systems), mouse anti‐human caspase‐3 (#31A1067, Novus Biologicals, LLC), and mouse anti‐human GAPDH (#MAB5718, R&D Systems) were used.
2.5. Gel filtration column chromatography
To characterize the size and concentration of intracellular HA, a 15 cm gel filtration or size exclusion chromatography column was set up using Sepharose 6B100 agarose beads (Sigma‐Aldrich) as previously described. 4 Twenty milliliters of PBS were used as the elution buffer and collected in glass tubes, 1 ml per tube, by gravity into 20 test tubes. Size standards were generated using both HA standards (1.5 M or 1010–1800 kDa, Lifecore Biomedical) and proteins (mouse thyroglobulin, 660 kDa, and bovine serum albumin, 66 kDa, Sigma‐Aldrich). The test tube at which each standard had the highest peak elution was identified by BCA assay or HA ELISA (R&D Systems). The elution of HMW HA (1.5 M or 1010–1800 kDa) peaked early at test tube 7. Elution of mouse thyroglobulin peaked at test tube 10, and elution of BSA peaked at test tube 13. For the samples, 50 μg of cell lysates was placed onto the Sepharose column prior to the elution buffer, 20 ml of PBS. The test tube with the peak elution and the concentration of the samples were identified and measured using HA ELISA (R&D Systems).
2.6. Data Analysis
Shapiro–Wilk normality test was used to determine if the values were from a Gaussian distribution. Data are shown as mean ± SD. For comparisons between two groups, an unpaired two‐tailed t test was used. For comparisons between multiple groups, analysis of variance (ANOVA) with post hoc Bonferroni’s correction was used. All statistical analysis was performed using GraphPad Prism 8.4.3 for OS X (GraphPad Software, www.graphpad.com).
3. RESULTS
3.1. Therapeutic effects of HMW HA on lung protein permeability across HLMVEC
Using a transwell co‐culture system, 50 ng/ml of cytomix increased protein permeability across HLMVEC at 24 h. The simultaneous administration of 100 μg/ml of HMW HA, unlike LMW HA, significantly improved protein permeability (Figure 1A). LDH activity, used as a measure of cell death, increased 24 h after the addition of cytomix. However, the administration of neither HMW HA nor LMW HA altered cell cytotoxicity (Figure 1B). Fluorescence microscopy for F‐actin demonstrated that cytomix induced “actin stress fiber” formation, resulting in gap formations between cells (Figure 1C). The simultaneous administration of HMW HA, unlike LMW HA, increased the distribution of F‐actin along the cell periphery and reduced gap formations between cells. The expression of CD44, a receptor for HA, was detected by Western blot analyses in HLMVEC. Injury with cytomix significantly increased CD44 expression (Figure 1D), which was further increased with HMW HA coincubation at 12 h. The therapeutic effects of HMW HA on protein permeability across HLMVEC were blocked by CD44 but not TLR4 neutralization (Figure 1E). As controls, incubation of uninjured HLMVEC with either HMW or LMW HA had no significant effect on protein permeability (Figure S1).
FIGURE 1.

Exogenous administration of HMW HA restored protein permeability across HLMVEC following an inflammatory insult. (A) Using a transwell coculture system, cytomix 50 ng/ml increased protein permeability across HLMVEC at 24 h. The addition of 100 μg/ml HMW HA but not LMW HA partially restored the permeability. Data are presented as mean ± SD. **p < 0.01, ****p < 0.0001; n = 6. (B) LDH levels increased 24 h after the addition of cytomix. Neither HMW HA nor LMW HA improved cell cytotoxicity. Data are presented as mean ± SD. ***p < 0.001, ****p < 0.0001; N = 12 per treatment group. (C) Fluorescence microscopy demonstrated actin stress fiber and gap formations in HLMVEC following cytomix‐induced injury. The addition of HMW HA partially restored the peripheral distribution of actin. Green = F‐actin, Blue = DAPI (nuclei), Yellow Arrows = Gap formations between cells. (D) Cytomix increased CD44 protein expression by HLMVEC, which was further increased with HMW HA at 12 h. Data are presented as mean ± SD. *p < 0.05, ***p < 0.001, ****p < 0.0001; N = 3. (E) Simultaneous addition of anti‐CD44 Ab but not anti‐TLR4 Ab eliminated the therapeutic effects of HMW HA on protein permeability across cytomix‐injured HLMVEC. Data are presented as mean ± SD. *p < 0.05, ****p < 0.0001; N = 9. Graphs were created using GraphPad Prism 8.4.3 for OS X (GraphPad Software, www.graphpad.com).
3.2. Effect of HMW HA on the expression and distribution of ZO‐1 and VE‐Cadherin among injured HLMVEC
Cytomix significantly decreased the protein expression of both ZO‐1 and VE‐cadherin among HLMVEC at 24 h (Figure 2A), which was partially attenuated with HMW or LMW HA administration. By fluorescence microscopy, cytomix reduced the distribution of both ZO‐1 and VE‐cadherin from the cell periphery and away from areas of cell–cell contact, which was partially attenuated with either HMW or LMW HA administration (Figure 2B). As controls, incubation of uninjured HLMVEC with either HMW or LMW HA had no significant effect on protein levels of ZO‐1 or VE‐Cadherin (Figure S1).
FIGURE 2.
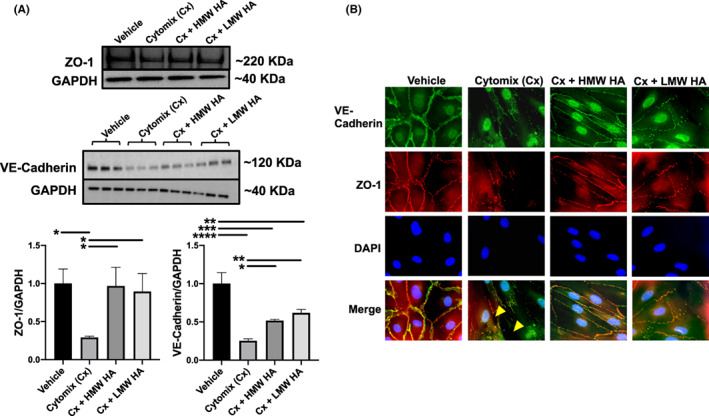
Effect of cytomix with or without hyaluronic acid on tight and adherens junctions on HLMVEC. (A) By Western blot, cytomix decreased the protein levels of both ZO‐1 and VE‐Cadherin in HLMVEC at 24 h. The administration of both HMW and LMW HA restored the protein levels of both junctional proteins. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; N = 3. (B) By fluorescence microscopy, cytomix decreased the overall expression of ZO‐1 and VE‐Cadherin and the distribution of the proteins away from cell–cell junctions. Large gap formations between cells were evident (yellow arrows). Administration of HMW HA largely restored the distribution of both ZO‐1 and VE‐cadherin to cell–cell junctions. Similar to the Western blot analyses, LMW HA also partially restored the loss of ZO‐1 and VE‐cadherin at the cell–cell junctions but to a lesser extent. Graphs were created using GraphPad Prism 8.4.3 for OS X (GraphPad Software, www.graphpad.com).
3.3. Effect of HMW HA on the expression of hyaluronic acid synthase among injured HLMVEC
HAS1 mRNA was not detected in HLMVEC. HAS2 mRNA expression was increased by cytomix over 24 h (Figure 3A). At 12 h, HMW HA administration further increased HAS2 mRNA expression, whereas LMW HA administration suppressed the increase in HAS2 mRNA expression. After 24 h, neither HMW HA nor LMW HA changed the expression of HAS2 mRNA. HAS3 mRNA increased after the addition of cytomix (Figure 3B). Neither HMW HA nor LMW HA changed the expression of HAS3 mRNA.
FIGURE 3.

Effect of cytomix with or without hyaluronic acid on HAS mRNA and HA Intracellular Levels in HLMVEC. (A) Cytomix injury increased HAS2 mRNA levels over 24 h. The addition of HMW HA further increased HAS2 mRNA expression at 12 h. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; N = 6 for PCR at 4 and 24 h. N = 12 for PCR at 12 h. (B) Cytomix increased HAS3 mRNA levels over 24 h. There was no effect with the addition of HMW or LMW HA. Data are presented as mean ± SD. **p < 0.01, ***p < 0.001, ****p < 0.0001; N = 6 for PCR at 4 and 24 h. N = 12 for PCR at 12 h. (C) The size and concentration of intracellular HA were measured in HLMVEC injured with cytomix with or without exogenous HMW or LMW HA administration by gel filtration column chromatography and ELISA. Cytomix decreased the intracellular levels of HA (both HMW and LMW) in HLMVEC. The addition of HMW HA dramatically increased the total expression of intracellular HA, predominantly HMW in size. The level of HMW HA in test tube #7 was approximately 7× the levels measured in the vehicle. The addition of LMW HA had a modest effect in restoring total intracellular HA but was dramatically lower than in HLMVEC treated with HMW HA. Data are presented as mean ± SD. N = 3. As controls, HA (1.0–1.8 MDa) eluted out at test tube #7, thyroglobulin (660 kDa) at test tube #10, and bovine serum albumin (66 kDa) at test tube #13. Graphs were created using GraphPad Prism 8.4.3 for OS X (GraphPad Software, www.graphpad.com)
To confirm the correlation between HAS gene expression and HA synthesis among injured HLMVEC, we measured the size distribution and intracellular level of the glycosaminoglycan using gel filtration column chromatography and ELISA (Figure 3C). As seen in our previous publication, 4 the elution of HMW HA peaked early at test tube 7 by gel filtration chromatography. The administration of cytomix largely eliminated the expression of the glycosaminoglycan at 24 h. In corroboration with the qPCR data on HAS2 expression, the simultaneous addition of HMW HA significantly increased the intracellular expression of HMW HA (Figure 3C).
3.4. Effect of HMW HA on the Phosphorylation of AKT Among Injured HLMVEC
The phosphorylation of AKT was significantly increased at 4 h after the addition of cytomix, which was further increased by HMW HA administration but not LMW HA (Figure S2). Although there was no decrease in cell cytotoxicity with HA administration, both HMW and LMW HA decreased active Caspase 3 expression at 4 h after the addition of cytomix (Figure S3).
To confirm whether these protein expressions were regulated via CD44, HLMVEC were incubated with a CD44 neutralizing antibody (IM7) for 4 h with HMW HA or LMW HA. The phosphorylation of AKT and the activation of Caspase‐3 by HMW HA were blocked by CD44 neutralization, whereas LMW HA had no effect (Figures S2 and S3).
3.5. Effect of 4‐MU, an inhibitor of HAS, on protein permeability across injured HLMVEC
We first examined the dose–response of 4‐MU, a HAS inhibitor, on both HAS2 mRNA expression and protein permeability across uninjured HLMVEC. The addition of 0.5 mM 4‐MU suppressed the expression of HAS2 mRNA but did not increase protein permeability (Figure 4A), whereas 1 mM of 4‐MU increased permeability at 24 h. Based on these results, 0.5 mM 4‐MU, which also did not affect cell cytotoxicity, was used in all subsequent experiments. The administration of 0.5 mM 4‐MU significantly suppressed the increase in HAS2 mRNA expression following the addition of cytomix (Figure 4B) and dramatically increased the permeability across HLMVEC. Fluorescence microscopy demonstrated that 4‐MU exacerbated F‐actin stress fiber formation and the formation of gaps between cells (Figure 4C). The addition of HMW HA had no therapeutic effects on protein permeability across HLMVEC, which may be explained by the lack of effect of HMW HA on HAS2 mRNA at 24 h. Despite the lack of therapeutic effects of HMW HA on protein permeability, HMW HA partially restored F‐actin, ZO‐1, and VE‐cadherin peripheral distribution by fluorescence microscopy and ZO‐1 and VE‐cadherin protein levels by Western blot analyses (Figure 4D–E). Furthermore, 4‐MU suppressed CD44 protein expression and the phosphorylation of AKT following the addition of cytomix (Figure 4F). HMW HA partially reversed the decrease in expression of CD44 but did not affect the phosphorylation of AKT at 24 h.
FIGURE 4.

Effect of 4‐MU, a HAS inhibitor, with or without hyaluronic acid on HLMVEC Injured With an Inflammatory Insult. (A) The addition of 4‐MU significantly suppressed the expression of HAS2 mRNA in HLMVEC in a dose‐dependent manner over 24 h. Only 4‐MU at a concentration of 1.0 mM led to an increase in protein permeability, whereas there were no significant differences with a 4‐MU concentration of 0.1–0.5 mM on permeability compared with vehicle control. Data are presented as mean ± SD. ****p < 0.0001; N = 3–6 for PCR and N = 9 for permeability experiments. (B) Addition of 4‐MU 0.5 mM with cytomix further decreased HAS2 mRNA expression and increased protein permeability across HLMVEC. There was no restorative effect of HMW HA on the decrease in HAS2 mRNA or increase in protein permeability. Data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; N = 3–6 for PCR and N = 9 for Permeability experiments. (C–E) However, by fluorescence microscopy and Western blot analyses, the addition of HMW HA partially decreased F‐actin stress fiber and increased both ZO‐1 and VE‐Cadherin total protein levels and distribution to cell–cell junctions in HLMVEC injured with both cytomix and 4‐MU 0.5 mM. Yellow Arrows = Gap formations between cells. Data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001; N = 3. (F) 4‐MU further suppressed CD44 protein expression and the phosphorylation of AKT after the addition of cytomix in HLMVEC. Only the addition of HMW HA partially increased the expression of CD44 at 24 h but not the phosphorylation of AKT. Data are presented as mean ± SD. *p < 0.05, **p < 0.01; N = 3. Individual p values are the statistical comparison between two groups by Student’s t test. Graphs were created using GraphPad Prism 8.4.3 for OS X (GraphPad Software, www.graphpad.com)
3.6. Effect of HMW HA on the expressions of high‐mobility group AT‐hook 2 and HAS2 antisense RNA 1 among injured HLMVEC
To examine the expressions of HAS2 Antisense RNA 1 (HAS2‐AS1) and high‐mobility Group AT‐hook 2 (HMGA2), which are known HAS2 promotors, qPCR and western blot analysis were performed at 4, 12, and 24 h after the addition of cytomix with or without HMW or LMW HA. Following injury by cytomix, HAS2‐AS1 and HMGA2 levels were increased over 24 h. HMW HA did not alter these mRNA expressions, whereas LMW HA suppressed them at 4 h. For Western blot analysis, HMGA2 was increased by cytomix over 24 h. At 4 h, HMW HA and LMW HA suppressed the increase of HMGA2. Interestingly at 12 h, HMGA2 expression was upregulated by HMW HA. The neutralization of CD44 blocked the decrease of HMGA2 in the HMW HA‐treated group but not the LMW HA‐treated group at 4 h (Figure S4). At 12 and 24 h, the upregulation of HMGA2 was suppressed by the neutralization of CD44 in both HMW HA‐ and LMW HA‐treated groups.
4. DISCUSSION
The major findings of our current study are (1) Exogenous HMW HA restored protein permeability across HLMVEC following an inflammatory injury, and this therapeutic effect was blocked by the addition of an anti‐CD44 Ab (Figure 1); (2) Neither HMW HA nor LMW HA altered HLMVEC cytotoxicity caused by cytomix at 24 h; (3) HMW HA, but not LMW HA, prevented actin stress fiber and intercellular gap formations (Figures 1); (4) By qPCR, incubation with cytomix upregulated the expression of HAS2, which was further increased with exogenous HMW HA, whereas exogenous LMW HA decreased HAS2 mRNA expression (Figure 3); (5) by gel filtration chromatography and ELISA, HMW HA increased intracellular HMW HA levels, unlike LMW HA (Figure 3); (6) the phosphorylation of AKT after cytomix‐induced injury was further increased with HMW HA administration, possibly leading to the inhibition of caspase‐3 activation (Figures S2 and S3); these effects were blocked by an anti‐CD44 Ab; (7) the addition of 4‐MU, a HAS inhibitor, dramatically exacerbated the protein permeability caused by cytomix. Although HMW HA improved ZO‐1 and VE‐cadherin protein expression and peripheral distribution, HMW HA did not restore protein permeability across HLMVEC following injury with cytomix and 4‐MU. However, HMW HA administration did improve CD44 protein expression (Figure 4); and (8) exogenous HMW HA upregulated not only HAS2 but HAS2‐AS1 and HMGA2, which was modulated partially thorough CD44 (Figure S4).
Exogenous administration of HMW HA suppressed the increase in protein permeability across HLMVEC caused by an inflammatory injury, which was blocked with CD44 neutralization, suggesting that the therapeutic effects were CD44 receptor mediated. Xu et al. found a similar beneficial effect with HA in LPS‐induced ALI in mice in terms of influx of inflammatory cells and cytokine levels in the injured alveolus via activation of the TLR4 receptor. 28 However, in the current study, neutralization of TLR4 did not suppress the therapeutic effects of HMW HA on protein permeability, which may be a result of different ALI endpoints and the size of HA administered.
CD44 plays a significant role in regulating tight and adherens junctions, which are critical for maintaining endothelial barrier integrity. 29 , 30 , 31 , 32 In the current study, both HMW HA and LMW HA increased the total expression and peripheral distribution of ZO‐1 and VE‐cadherin, although LMW HA did not improve protein permeability. Interestingly, fluorescence microscopy demonstrated that HMW HA, but not LMW HA, reduced actin stress fiber formation which is the predominant cause of cell contraction, formation of gap formations, and permeability. 33 More importantly, for the first time in the literature, our results demonstrated that HMW HA, but not LMW HA, increased CD44, HAS2, and subsequently intracellular HMW HA expression following injury, which may be critical for the reparative effect. The increase in intracellular HMW HA levels was an unexpected finding given that the normal feedback response to exogenous HMW HA would be downregulation of HAS. We speculate that the increase in AKT phosphorylation with HMW HA may drive the increase in HMW HA synthesis. 34 HA is an important component of the glycocalyx layer, which covers the surface of the vascular endothelium. 5 , 6 , 7 During ALI, the glycocalyx layer on the endothelium is degraded, exposing the endothelium to the cytotoxic effects of cytokines. By increasing HAS2 expression and intracellular HMW HA synthesis via activation of CD44, 35 exogenous HMW HA appeared to restore the glycocalyx layer.
Exogenous LMW HA is not studied as a therapeutic or administered in ALI models because of its known pro‐permeability and pro‐inflammatory effects. 36 Hence, there is an insufficient amount of published data describing the effects of LMW HA on biological and cellular processes. However, in earlier publications prior to the characterization of LMW HA, administration of exogenous HMW or LMW HA as therapy was found to behave in both similar and different ways in terms of ALI endpoints. For example, in sepsis‐induced ALI following LPS infusion in mechanically ventilated rats, Liu et al. found that both intravenous HMW (1600 kDa) and LMW (35 kDa) HA reduced neutrophil intravasation, MPO activity, and inflammation in the lung but only HMW HA reduced both neutrophil and monocyte infiltration and lung injury score by histology. 37 It remains unclear why HA behaves differently based on its molecular weight. Some investigators have speculated that the size of the HA can influence the binding of HA to cognate receptors such as CD44 which can determine the effect size. 20
Previous studies demonstrated a relationship between HAS2 levels and AKT/PI3K signaling. Maroski et al. showed that vascular shear stress across the endothelium increased both HAS2 expression and HMW HA synthesis, which was suppressed with an AKT inhibitor. 34 In cancer, multiple investigators found that CD44 activation led to AKT signaling, which was suppressed with HAS inhibitors. 38 , 39 , 40 These studies suggested that there was a positive feedback mechanism between HAS and AKT signaling. 41 In the current study, exogenous administration of HMW HA, but not LMW HA, increased the phosphorylation of AKT in injured HLMVEC, suggesting that the upregulation of HAS2 by HMW HA was in part mediated by AKT signaling via CD44. Although there were no beneficial effects by HMW HA on cell cytotoxicity at 24 h possibly due to the severity of the injury at earlier timepoints, the results also demonstrated that exogenous HMW HA decreased active caspase‐3 levels, which was blocked by CD44 neutralization; Wang et al. found that cytomix at 30 ng/mL induced significant levels of apoptosis in HLMVECs as early as 5 h of exposure. 42 AKT activation can inhibit caspase‐3 activation, resulting in a decrease in apoptosis 43 , 44 (Figure 5).
FIGURE 5.

Schematic of the mechanisms underlying the therapeutic effects of HMW HA on injured human lung microvascular endothelial cells. Cytomix, a mixture of IL‐1β, TNFα, and IFNγ often used as a surrogate for acute respiratory distress syndrome pulmonary edema fluid, caused an increase in protein permeability across HLMVEC which was associated with an increase in apoptosis/LDH release and actin stress fiber and gap formations between cells. Cytomix was also capable of breaking down HMW HA into LMW HA further exacerbating the injury. In response to cytomix, AKT phosphorylation and HAS2 expression were increased in HLMVECs as an inherent reparative effect although there was no restoration of intracellular HMW HA levels or protein permeability. Surprisingly, exogenous HMW HA administration increased HAS2 expression further after cytomix through CD44, leading to an increase in intracellular HMW HA synthesis and restoration of protein permeability. This finding was unexpected given normal feedback inhibition seen with receptors and end‐products and may result from an increase in AKT phosphorylation from HMW HA leading to increased HAS2 expression. Although exogenous LMW HA administration suppressed Caspase‐3 activation in the current study, LMW HA is known to increase inflammation, apoptosis, and permeability in endothelial cells whether through a direct effect or through increased release of cytokines or through inhibition of HMW HA binding to CD44 as a competitive antagonist
Additional experiments using 4‐MU, a pan HAS inhibitor, were performed to confirm the importance of increased HAS2 expression in the restoration of protein permeability with HMW HA 26 , 45 ; HAS inhibition by 4‐MU was previously reported to decrease the glycocalyx layer in vascular endothelium. 46 In the current study, we demonstrated that suppression of HAS2 by 4‐MU resulted in further exacerbation of permeability with or without cytomix, which was not alleviated with HMW HA incubation. The effect of 4‐MU on permeability was also associated with reduced ZO‐1, VE‐Cadherin, and CD44 protein expression. Surprisingly, exogenous HMW HA improved the peripheral distribution of the tight and adherens junction proteins by Western blot analyses and fluorescence microscopy. In addition, incubation with 4‐MU significantly suppressed the phosphorylation of AKT, which was not restored with HMW HA treatment. But, HMW HA did increase CD44 expression. Although AKT activation can lead to upregulation of CD44, exogenous HMW HA may directly activate CD44. 27
We next examined the role of HMGA2 and HAS2‐AS1 expressions on HAS2 regulation. 47 HMGA2 is a known oncogene, which has several known effects on the cell cycle, DNA damage repair, and apoptosis. 48 Both HMGA2 and HAS2‐AS1 are known promotors of HAS2. 49 , 50 Our results demonstrated that cytomix increased the expression of not only HAS2 but also HMGA2 and HAS2‐AS1 in HLMVEC, which was further increased with exogenous HMW HA via CD44. Interestingly, the expression of HMGA2 is associated with apoptosis and can be regulated by AKT and Caspase‐3. 48 Further studies are underway to study the relationship between HMGA2/HAS2‐AS1 and HAS2 and the progression of ARDS.
There are limitations to the current study as follows: (1) The differential expression of HAS2 by HMW and LMW HA is not completely understood. 51 HA may have different affinities for the CD44 receptor depending on its MW and concentration. 8 , 52 In addition, HMW and LMW HA may also have different signaling effects on CD44 53 ; (2) 4‐MU can inhibit HAS1‐3. HAS3 is known to increase LMW HA synthesis, leading to the induction of a pro‐inflammatory response. 54 , 55 In LPS‐induced ALI, McKallip et al. found that 4‐MU administration improved pulmonary vascular permeability and reduced inflammation. 56 In addition, Li et al. found that 4‐MU administration suppressed LPS‐induced TLR activation, leading to the decrease of cytokine expression, in corneal stromal cells. 57 And in a septic ventilated rate model, Mrabat et al. found that inhibition of HAS3 improved ALI. 58 Whereas in our study, administration of 4‐MU exacerbated endothelial protein permeability. The discrepancy with our results may reflect differences in the model used, the timing of 4‐MU administration, and the differential effect of 4‐MU on HAS1‐3 expressions depending on the dose. (3) And although the use of HMW HA as a therapeutic for ALI is promising, the findings from the in vitro studies must be confirmed in vivo to definitively establish the mechanism. Studies are ongoing. For example, Baljinnyam et al. found that HMW HA reduced lung tissue damage and mortality in septic syndecan‐1 KO mice, in part by reducing vascular hyper‐permeability and neutrophil migration. 59 Syndecan‐1 is a core protein in heparan sulfate proteoglycan, a major component of the endothelial glycocalyx layer. Hence, further investigations involving the role of HAS1‐3 in ALI in vivo are warranted, especially on protein permeability.
In conclusion, exogenous HMW HA but not LMW HA restored protein permeability across HLMVEC injured by an inflammatory insult in part through interaction with CD44 receptor on the endothelium with subsequent upregulation of HAS2, the predominant enzyme involved in HMW HA synthesis. The upregulation of HAS2 was critical for the therapeutic effect of HMW HA on protein permeability in part regulated by AKT phosphorylation, Caspase‐3 activation, and HMGA2 modulation via CD44 (Figure 5). There is currently no pharmacological therapy that reduces mortality in patients who develop ARDS. Based on the current findings, exogenous HMW HA may be a potential therapeutic to reduce lung protein permeability, a key pathological feature in the injured alveolus. Thus, further studies of HMW HA are warranted in vivo such as in a large animal model of pneumonia and/or sepsis to complement the existing preclinical studies in small animals for possible translation to clinical trials.
CREDIT STATEMENT
Shinji Sugita: Designed research, performed research, analyzed data, wrote the paper. Yoshifumi Naito: Performed research, contributed new reagents or analytic tools. Li Zhou: Performed research, contributed new reagents or analytic tools. Hongli He: Performed research, contributed new reagents or analytic tools. Qi Hao: Performed research, contributed new reagents or analytic tools. Atsuhiro Sakamoto: Performed research, contributed new reagents or analytic tools. Jae W. Lee: Designed research, performed research, analyzed data, wrote the paper.
FUNDING INFORMATION
The National Institute of Health National Heart, Lung, and Blood Institute (grant number HL 113022 and HL 148781, Dr. J. W. Lee).
Supporting information
Supplemental Figure 1
Figure S2.
Figure S3.
Figure S4.
Appendix S1
Sugita S, Naito Y, Zhou L, et al.. Hyaluronic acid restored protein permeability across injured human lung microvascular endothelial cells. FASEB BioAdvances. 2022;4(9):619‐631. doi: 10.1096/fba.2022-00006
REFERENCES
- 1. Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562‐572. [DOI] [PubMed] [Google Scholar]
- 2. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731‐2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788‐800. [DOI] [PubMed] [Google Scholar]
- 4. Liu A, Park JH, Zhang X, et al. Therapeutic effects of hyaluronic acid in bacterial pneumonia in ex vivo perfused human lungs. Am J Respir Crit Care Med. 2019;200:1234‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300‐310. [DOI] [PubMed] [Google Scholar]
- 6. Wheeler‐Jones CP, Farrar CE, Pitsillides AA. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Curr Opin Investig Drugs. 2010;11:997‐1006. [PubMed] [Google Scholar]
- 7. Pillinger NL, Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth Intensive Care. 2017;45:295‐307. [DOI] [PubMed] [Google Scholar]
- 8. Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size‐dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286:2883‐2908. [DOI] [PubMed] [Google Scholar]
- 9. Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis. 2011;1:200‐213. [PMC free article] [PubMed] [Google Scholar]
- 10. Eldridge L, Moldobaeva A, Wagner EM. Increased hyaluronan fragmentation during pulmonary ischemia. Am J Physiol Lung Cell Mol Physiol. 2011;301:L782‐L788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins SL, Black KE, Chan‐Li Y, et al. Hyaluronan fragments promote inflammation by down‐regulating the anti‐inflammatory A2a receptor. Am J Respir Cell Mol Biol. 2011;45:675‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater Sci Eng. 2015;1:481‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu C, Shi Q, Zhang L, Zhao H. High molecular weight hyaluronan attenuates fine particulate matter‐induced acute lung injury through inhibition of ROS‐ASK1‐p38/JNK‐mediated epithelial apoptosis. Environ Toxicol Pharmacol. 2018;59:190‐198. [DOI] [PubMed] [Google Scholar]
- 15. Mascarenhas MM, Day RM, Ochoa CD, et al. Low molecular weight hyaluronan from stretched lung enhances interleukin‐8 expression. Am J Respir Cell Mol Biol. 2004;30:51‐60. [DOI] [PubMed] [Google Scholar]
- 16. Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967‐26975. [DOI] [PubMed] [Google Scholar]
- 17. Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan‐binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol. 2011;301:L137‐L147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vigetti D, Genasetti A, Karousou E, et al. Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor‐kappaB (NF‐kappaB) pathway. J Biol Chem. 2010;285:24639‐24645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viola M, Karousou E, D'Angelo ML, et al. Regulated hyaluronan synthesis by vascular cells. Int J Cell Biol. 2015;2015:208303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by toll‐like receptors and hyaluronan. Nat Med. 2005;11:1173‐1179. [DOI] [PubMed] [Google Scholar]
- 21. Singleton PA, Mirzapoiazova T, Guo Y, et al. High‐molecular‐weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol. 2010;299:L639‐L651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1‐phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381‐34393. [DOI] [PubMed] [Google Scholar]
- 23. Hu S, Park J, Liu A, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018;7:615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin‐1. J Biol Chem. 2010;285:26211‐26222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kultti A, Pasonen‐Seppanen S, Jauhiainen M, et al. 4‐methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP‐glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914‐1923. [DOI] [PubMed] [Google Scholar]
- 27. Lokeshwar VB, Lopez LE, Munoz D, et al. Antitumor activity of hyaluronic acid synthesis inhibitor 4‐methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613‐2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu C, Chen G, Yang W, et al. Hyaluronan ameliorates LPS‐induced acute lung injury in mice via toll‐like receptor (TLR) 4‐dependent signaling pathways. Int Immunopharmacol. 2015;28:1050‐1058. [DOI] [PubMed] [Google Scholar]
- 29. Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71:462‐473. [DOI] [PubMed] [Google Scholar]
- 30. Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4:535‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirschner N, Haftek M, Niessen CM, et al. CD44 regulates tight‐junction assembly and barrier function. J Invest Dermatol. 2011;131:932‐943. [DOI] [PubMed] [Google Scholar]
- 32. Tsuneki M, Madri JA. CD44 regulation of endothelial cell proliferation and apoptosis via modulation of CD31 and VE‐cadherin expression. J Biol Chem. 2014;289:5357‐5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen Q, Wu MH, Yuan SY. Endothelial contractile cytoskeleton and microvascular permeability. Cell Health Cytoskelet. 2009;2009:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maroski J, Vorderwulbecke BJ, Fiedorowicz K, et al. Shear stress increases endothelial hyaluronan synthase 2 and hyaluronan synthesis especially in regard to an atheroprotective flow profile. Exp Physiol. 2011;96:977‐986. [DOI] [PubMed] [Google Scholar]
- 35. van der Windt GJ, Schouten M, Zeerleder S, Florquin S, van der Poll T. CD44 is protective during hyperoxia‐induced lung injury. Am J Respir Cell Mol Biol. 2011;44:377‐383. [DOI] [PubMed] [Google Scholar]
- 36. Mambetsariev N, Mirzapoiazova T, Mambetsariev B, et al. Hyaluronic acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler Thromb Vasc Biol. 2010;30:483‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu YY, Lee CH, Dedaj R, et al. High molecular weight hyaluronan: a possible new treatment for sepsis‐induced lung injury – a preclinical study in mechanically ventilated rats. Crit Care. 2008;12:R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Price ZK, Lokman NA, Ricciardelli C. Differing roles of hyaluronan molecular weight on cancer cell behavior and chemotherapy resistance. Cancers (Basel). 2018;10:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morera DS, Hennig MS, Talukder A, et al. Hyaluronic acid family in bladder cancer: potential prognostic biomarkers and therapeutic targets. Br J Cancer. 2017;117:1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yates TJ, Lopez LE, Lokeshwar SD, et al. Dietary supplement 4‐methylumbelliferone: an effective chemopreventive and therapeutic agent for prostate cancer. J Natl Cancer Inst. 2015;107:djv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu S, Cheng C. Akt signaling is sustained by a CD44 splice isoform‐mediated positive feedback loop. Cancer Res. 2017;77:3791‐3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang L, Mehta S, Ahmed Y, Wallace S, Pape MC, Gill SE. Differential mechanisms of septic human pulmonary microvascular endothelial cell barrier dysfunction depending on the presence of neutrophils. Front Immunol. 2018;9:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu J, Tu F, Yao W, et al. Conserved miR‐26b enhances ovarian granulosa cell apoptosis through HAS2‐HA‐CD44‐Caspase‐3 pathway by targeting HAS2. Sci Rep. 2016;6:21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cui W, Leng B, Liu W, Wang G. Suppression of apoptosis in human umbilical vein endothelial cells (HUVECs) by klotho protein is associated with reduced endoplasmic reticulum oxidative stress and activation of the PI3K/AKT pathway. Med Sci Monit. 2018;24:8489‐8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nagy N, Kuipers HF, Frymoyer AR, et al. 4‐methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol. 2015;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nagy N, Freudenberger T, Melchior‐Becker A, et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122:2313‐2322. [DOI] [PubMed] [Google Scholar]
- 47. Kolliopoulos C, Lin CY, Heldin CH, Moustakas A, Heldin P. Has2 natural antisense RNA and Hmga2 promote Has2 expression during TGFbeta‐induced EMT in breast cancer. Matrix Biol. 2019;80:29‐45. [DOI] [PubMed] [Google Scholar]
- 48. Zhang S, Mo Q, Wang X. Oncological role of HMGA2 (review). Int J Oncol. 2019;55:775‐788. [DOI] [PubMed] [Google Scholar]
- 49. Passi A, Vigetti D, Buraschi S, Iozzo RV. Dissecting the role of hyaluronan synthases in the tumor microenvironment. FEBS J. 2019;286:2937‐2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yung Y, Ophir L, Yerushalmi GM, Baum M, Hourvitz A, Maman E. HAS2‐AS1 is a novel LH/hCG target gene regulating HAS2 expression and enhancing cumulus cells migration. J Ovarian Res. 2019;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heldin P, Lin CY, Kolliopoulos C, Chen YH, Skandalis SS. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019;78‐79:100‐117. [DOI] [PubMed] [Google Scholar]
- 52. Li Z, Potts‐Kant EN, Garantziotis S, Foster WM, Hollingsworth JW. Hyaluronan signaling during ozone‐induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One. 2011;6:e27137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 53. Fuchs K, Hippe A, Schmaus A, Homey B, Sleeman JP, Orian‐Rousseau V. Opposing effects of high‐ and low‐molecular weight hyaluronan on CXCL12‐induced CXCR4 signaling depend on CD44. Cell Death Dis. 2013;4:e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Homann S, Grandoch M, Kiene LS, et al. Hyaluronan synthase 3 promotes plaque inflammation and atheroprogression. Matrix Biol. 2018;66:67‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pure E, Krolikoski M, Monslow J. Role for hyaluronan synthase 3 in the response to vascular injury. Arterioscler Thromb Vasc Biol. 2016;36:224‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKallip RJ, Ban H, Uchakina ON. Treatment with the hyaluronic acid synthesis inhibitor 4‐methylumbelliferone suppresses LPS‐induced lung inflammation. Inflammation. 2015;38:1250‐1259. [DOI] [PubMed] [Google Scholar]
- 57. Li F, Hao P, Liu G, et al. Effects of 4‐methylumbelliferone and high molecular weight hyaluronic acid on the inflammation of corneal stromal cells induced by LPS. Graefes Arch Clin Exp Ophthalmol. 2017;255:559‐566. [DOI] [PubMed] [Google Scholar]
- 58. Mrabat H, Beagle J, Hang Z, Garg HG, Hales CA, Quinn DA. Inhibition of HA synthase 3 mRNA expression, with a phosphodiesterase 3 inhibitor, blocks lung injury in a septic ventilated rat model. Lung. 2009;187:233‐239. [DOI] [PubMed] [Google Scholar]
- 59. Baljinnyam T, Radnaa E, Ouellette CM, et al. High molecular weight sodium hyaluronate improves survival of syndecan‐1‐deficient septic mice by inhibiting neutrophil migration. PLoS One. 2021;16:e0250327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
Figure S2.
Figure S3.
Figure S4.
Appendix S1


