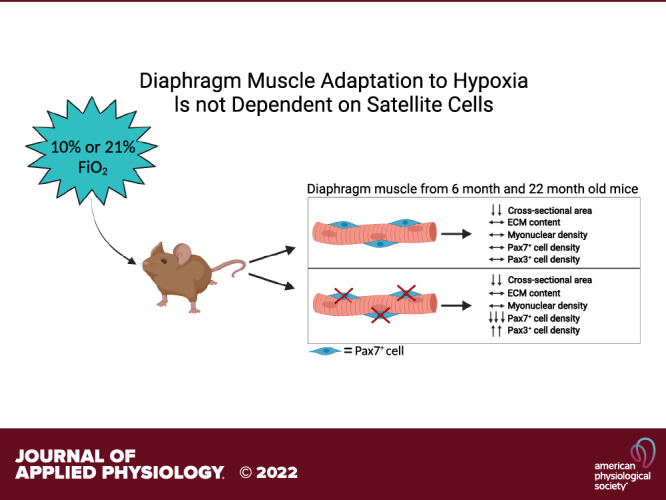
Keywords: aging, diaphragm, hypoxia, Pax3, satellite cells
Abstract
The diaphragm is the main skeletal muscle responsible for inspiration and is susceptible to age-associated decline in function and morphology. Satellite cells in diaphragm fuse into unperturbed muscle fibers throughout life, yet their role in adaptations to hypoxia in diaphragm is unknown. Given their continual fusion, we hypothesize that satellite cell depletion will negatively impact adaptations to hypoxia in the diaphragm, particularly with aging. We used the Pax7CreER/CreER:R26RDTA/DTA genetic mouse model of inducible satellite cell depletion to investigate diaphragm responses to hypoxia in adult (6 mo) and aged (22 mo) male mice. The mice were subjected to normobaric hypoxia at 10% or normoxia for 4 wk. We showed that satellite cell depletion had no effect on diaphragm muscle fiber cross-sectional area, fiber-type distribution, myonuclear density, or regulation of extracellular matrix in either adult or aged mice. Furthermore, we showed lower muscle fiber cross-sectional area with hypoxia and age (main effects), while extracellular matrix content was higher and satellite cell abundance was lower with age (main effect) in diaphragm. Lastly, a greater number of Pax3-mRNA+ cells was observed in diaphragm muscle of satellite cell-depleted mice independent of hypoxia (main effect), potentially as a compensatory mechanism for the loss of satellite cells. We conclude that satellite cells are not required for diaphragm muscle adaptations to hypoxia in either adult or aged mice.
NEW & NOTEWORTHY Satellite cells show consistent fusion into diaphragm muscle fibers throughout life, suggesting a critical role in maintaining homeostasis. Here, we report identical diaphragm adaptations to hypoxia with and without satellite cells in adult and aged mice. In addition, we propose that the higher number of Pax3-positive cells in satellite cell-depleted diaphragm muscle acts as a compensatory mechanism.
INTRODUCTION
The diaphragm is an extremely important skeletal muscle given its critical role in ventilation during which it must contract throughout life. Age-associated changes occur in diaphragm, similar to limb muscles, including decreased force output, atrophy of fast-twitch muscle fibers, and decreased whole muscle power output (1–9). Identifying processes that underly age-associated changes in diaphragm muscle and its ability to adapt to external stressors will allow characterization of its susceptibility to functional impairments.
Skeletal muscle stem cells, also known as satellite cells, are indispensable for recovery of muscle damage, evidenced by a lack of regeneration in severely injured muscles of satellite cell-ablated mice (10–13). The role of satellite cells during muscle fiber hypertrophy and recovery from atrophy in limb muscles, however, remains debated (14–17); fusion of satellite cells occurs during hypertrophy but is not required (18–20). Satellite cell fusion with muscle fibers occurs throughout life, even in sedentary mice, suggesting a potential role for satellite cells in the maintenance of muscle fiber homeostasis with a particularly large degree of satellite cell fusion in diaphragm (21, 22). By contrast, lifelong satellite cell depletion does not induce accelerated sarcopenia in sedentary animals (23), questioning the role of satellite cells in muscle mass maintenance with age. Satellite cell fusion into muscle fibers was shown to be higher in diaphragm than limb muscles and therefore was deemed indispensable for maintaining diaphragm homeostasis throughout life (21).
Our previous work showed that satellite cells in sedentary mice and those during voluntary wheel-running conditions are not required for normal function or adaptation in diaphragm muscle in adult and aged mice (24). It was suggested that voluntary wheel running was not robust enough to challenge the diaphragm muscle and stimulate satellite cells to contribute to the adaptation (25). Therefore, in this study, we will use hypoxia as a stimulus to challenge the diaphragm; hypoxia induces a robust increase in ventilation, forcing adaptations that may require satellite cell activity (26, 27). In addition, chronic hypoxia influences satellite cell differentiation and proliferation capacity by affecting the expression of miR1 and miR206, as well as MyoD and notch signaling indicating the potential importance of satellite cells for the adaptation to hypoxia (28–31). Therefore, we hypothesized that satellite cell depletion would negatively impact adaptations to hypoxia in the diaphragm and these maladaptations would be exacerbated by age. The Pax7CreER/CreER:R26RDTA/DTA (Pax7-DTA) mouse model was used to deplete satellite cells in adult and aged mice housed in normobaric hypoxia chambers to induce hypoxia.
METHODS
Animal Model
The Pax7CreER/CreER mouse was crossed with a Rosa26DTA/DTA mouse generating the Pax7-DTA mouse on a C57BL6/129 background, which permits tamoxifen-inducible depletion of satellite cells, as previously described (19). All animal procedures were carried out in accordance with the institutional guidelines for care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Mice were maintained on a 14:10-h light-dark cycle, at room temperature 22 C, with food (irradiated Teklad 2918 rodent diet, Envigo, Indianapolis, IN) and water ad libitum.
Experimental Design
Adult male Pax7-DTA mice (4 mo, n = 9–12/group) were randomly assigned to either receive an intraperitoneal injection of tamoxifen at a dose of 2.5 mg/day for five consecutive days or vehicle control (15% ethanol in sunflower seed oil) as previously described (19). A washout period of at least 8 wk following the injections was used before any experimentation. At 6 (adult) and 22 (aged) mo of age, vehicle- or tamoxifen-treated mice were randomly assigned to normoxic or hypoxic groups. Adult and aged mice in the hypoxia group were first acclimated to the normobaric hypoxia chamber at 15% for 2 days, followed by 1 day at 13% and 11% and maintained at 10% for the remainder of the 4-wk period. A period of 4 wk and 10% was chosen as it has been shown that mitochondrial changes in diaphragm in response to this level of hypoxia take up to 4 wk to develop and this would also allow for changes in myosin heavy chain distribution to be present (32). Mice in the normoxia group were housed in regular cages at ambient Po2 and for the duration of the study. Cage changes occurred a total of three times over the 4-wk duration of the study with a maximum cage change time of 5 min. After 4 wk, mice were subjected to ultrasound analysis for in vivo diaphragm function and euthanized by sodium pentobarbital overdose (150 mg/kg). Blood was collected through cardiac puncture in a Microvette capillary blood collection tube (Sarstedt, Newton, NC) and red blood cell count (RBC), hemoglobin (HB), and hematocrit (HCT) were determined using the HEMA-Set3 reagent kit and the Drew Scientific Hemavet950 multispecies hematology analyzer (GMI, Ramsey, MN). In this study, we used a single end point where blood parameters were assessed. This was done to validate our hypoxia stimulus while avoiding hypohematic stress in the animals being studied. Costal diaphragm muscle strips were dissected, and one strip was covered in optimal cutting temperature compound (OCT) and snap frozen in liquid nitrogen-cooled isopentane and stored at −80°C until further analysis. One strip was fixed in 4% paraformaldehyde and used for single fiber muscle analysis.
Ultrasound Analysis of In Vivo Diaphragm Function in Conscious Mice
Ultrasound analysis was performed in a blinded fashion as previously described (24, 33). Mice were removed from hypoxia chambers, awake and in an upright position. Although these measures may not resemble true resting values or values observed under hypoxic conditions, this approach is much more translatable than conducting these measures while the mouse was anesthetized. The day before the scan, the abdominal hair was removed with depilatory cream (NairTM Church & Dwight Co. Trenton, NJ). The probe with ultrasonic gel was placed on the abdomen close to the xyphoid process pointing upward for diaphragm visualization using the VisualSonics Vevo 2100 Imaging System with MS-400 scan head (FUJIFILM VisualSonics Inc., Toronto, ON) at 38 MHz. M-mode images were captured of 10–50 respiratory cycles. Of those, 10–20 cycles were measured for breath rate, breath depth, and inspiration and expiration velocity.
Immunohistochemistry and Image Analysis
Immunohistochemistry was performed as previously described (24). Diaphragm was cut into 8-µm thick sections and air-dried for 1 h. Sections were fixed in 4% paraformaldehyde (PFA) followed by epitope retrieval using sodium citrate (10 mM, pH 6.5) at 92°C for 10 min. Sections were cooled to room temperature (RT) and endogenous peroxidase activity was quenched using 3% hydrogen peroxide in phosphate-buffered saline (PBS). Nonspecific binding was blocked using Mouse-on-Mouse block (Vector Laboratories, Burlingame, CA) and 1% bovine serum albumin (Thermo Fisher, Waltham, MA). Sections were incubated overnight at 4°C with Pax7 primary antibody (1:100, Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA). The next day, samples were incubated with an anti-mouse biotin-conjugated secondary antibody (1:1,000, Jackson ImmunoResearch, West Grove, PA) for 1 h. Signal amplification was performed using streptavidin-horseradish peroxidase (1:500, ThermoFisher) and Tyramide Signal Amplification (TSA) AlexaFluor 488 to visualize antibody binding. A biotin/streptavidin blocking kit (Vector Laboratories) was added to block unbound biotin/streptavidin. An anti-rabbit biotin-conjugated secondary antibody (1:1,000, Jackson Immunoresearch) was added for 1 h at RT and antibody-binding was visualized using AlexaFluor 594 streptavidin conjugate (ThermoFisher). Finally, sections were mounted and nuclei were stained using Vectashield antifade mounting media with DAPI (Vector Laboratories). Sections were imaged at ×20 magnification using a Zeiss upright fluorescent microscope (Zeiss AxioImager M1 Oberkochen, Germany). Satellite cells were identified as Pax7+/DAPI+ cells. Satellite cell number was expressed per myofiber. Dystrophin staining was used to quantify cross-sectional area (CSA) using Myovision, a fully automated muscle analysis software program (34).
Extracellular matrix (ECM) content was measured by detection of N-acetyl-d-glucosamine using AlexaFlour 555-conjugated wheat germ agglutinin (WGA) (1:50, Invitrogen). This protocol was performed as previously described (24). In brief, sections were fixed in 4% PFA, then incubated with WGA conjugate, and images were captured at ×20 magnification. Staining was quantified using the thresholding feature in AxioVision Rel software (Zeiss, Oberkochen, Germany). The area occupied by WGA was expressed relative to muscle fiber number as WGA index.
Frozen sections were incubated overnight with isotype-specific anti-mouse primary antibodies for myosin heavy chain (MyHC) I (1:75, IgG2B, BA.D5), MyHC IIa (supernatant, IgG1, SC.71), and MyHC IIx (supernatant, IgM, 6H1) from DSHB, along with laminin (1:100, Sigma-Aldrich, St. Louis, MO). Sections were then incubated with secondary antibodies (1:250, goat anti-mouse IgG2B Alexa fluor 647; 1:500, IgG1 Alexa fluor 488; 1:250, IgM Alexa fluor 555) from Invitrogen, all diluted in PBS, along with the secondary antibody for laminin (1:150, IgG AMCA, Vector). Sections were mounted with VectaShield mounting medium (Vector), and images were captured at ×20. Fiber-type distribution was quantified using Myovision as percent MyHC I, IIa, IIx, and IIb (no staining) (34). For all immunohistochemistry analyses we counted a minimum of 600 fibers for each animal and assessors were blinded to the experimental groups.
Single Muscle Fiber Myonuclear Analysis
Single muscle fiber analysis was performed as previously described (24). Costal diaphragm muscle was dissected, pinned down, and fixed at resting length in 4% PFA for 48 h. Tissue dissociation was performed in 40% NaOH for 2 h, single fibers were mechanically pulled apart, strained, and washed with PBS before staining with DAPI (1:10,000 in PBS, Invitrogen) for myonuclear visualization, with the assumption that most mononuclear cells were dissociated from the muscle fibers during the isolation steps. Nuclei that appeared to be loosely associated with the muscle fiber were not counted. Suspended fibers were dispersed onto a slide and mounted with VectaShield Fluorescent mounting media (Vector). Images were captured as z-stacks at ×20 magnification, and all fibers and nuclear measurements were made using Zeiss Microscopy’s Zen software. Twenty fibers from each animal were measured by a blinded trained rater to determine the number of myonuclei per defined fiber segment.
Fluorescent In Situ Hybridization for Pax3
Pax3 fluorescent in situ hybridization (FISH) was performed as previously described (24). Briefly, to detect Pax3 mRNA in diaphragm cross-sections, we adapted the Stellaris RNA FISH protocol from Biosearch Technologies (Petaluma, CA) using Stellaris RNA FISH hybridization buffer and Stellaris RNA FISH wash buffer A. RNA FISH probes were designed by Integrated DNA Technologies (Coralville, IA) and conjugated with a biotin tag. Diaphragm muscle was sectioned (8 µm) and immediately stored in 100% ethanol at −20°C for fixation and RNA preservation. Slides were rinsed, and RNAses were inactivated by incubating in 0.1% diethyl pyrocarbonate (DEPC) and H2O. Samples were then immersed in 4% PFA, washed in PBS, and incubated in wash buffer A for 5 min. Probes were added to the hybridization buffer to a concentration of 625 nM and added to the slides for 5 h at 37°C in a humidified chamber. Following incubation with probes, samples were washed in wash buffer A and incubated with streptavidin-HRP (Invitrogen). TSA Alexa Fluor 594 (1:100, Invitrogen) was used to visualize hybridization. Sections were mounted and nuclei were stained using VectaShield antifade mounting media with DAPI (Vector). Probes used were Pax3-1728, Pax3-3130, and a negative control (NC) scrambled probe:
Pax3-1728:
/5Biosg/mUmAmUmAmGmGmUmGmGmGmGmGmGmAmCmAmAmUmAmG
Pax3-3130:
/5Biosg/mCmCmUmCmUmAmCmAmUmCmAmUmAmCmCmCmUmUmCmC
NC (negative control):/5Biosg/mGmCmGmAmCmUmAmUmAmCmGmCmGmCmAmAmUmAmUmG
Pax3+ cells were identified as Pax3+/DAPI+ and were expressed per number of muscle fibers.
Statistical Analysis
Data were analyzed with GraphPad Prism software (GraphPad, San Diego, CA). Data were checked for normality, and those not normally distributed underwent a log transformation. Parametric analyses were used for all measures. Three-way ANOVAs were used to determine main effects for age, followed by a two-way ANOVA to test all other comparisons in both ages. If a significant effect or interaction was detected, Šídák’s multiple comparison post hoc test was used to determine the source of the significance. Statistical significance was accepted at P ≤ 0.05. Data are reported as means ± SE.
RESULTS
Diaphragm Satellite Cell Density Is Lower with Age but Not Hypoxia
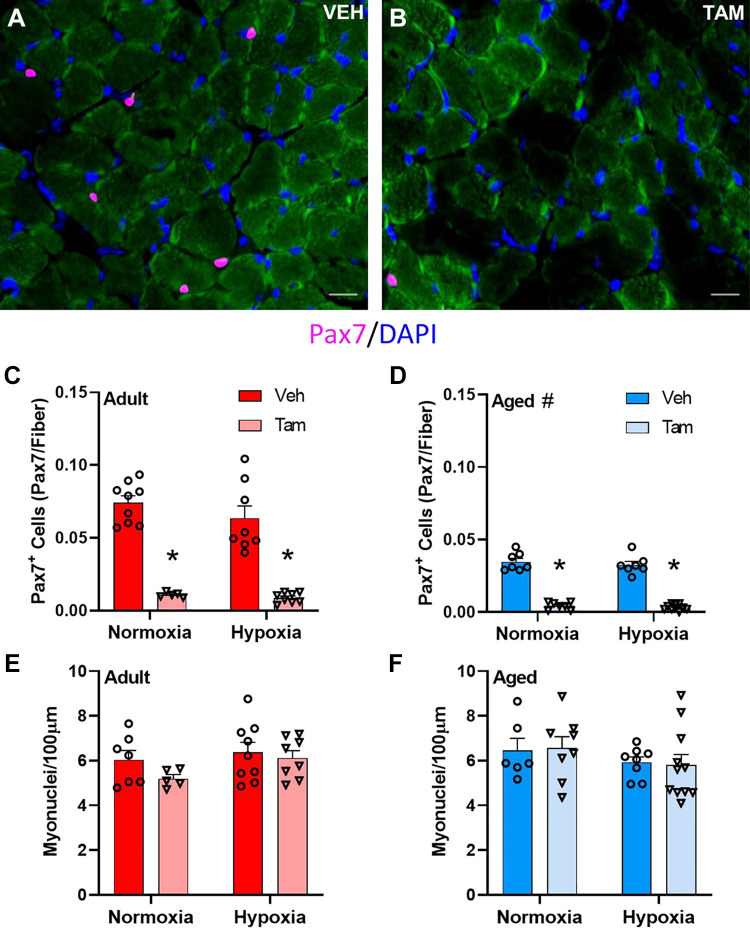
Representative images of diaphragm muscle cross sections from vehicle- and tamoxifen-treated mice are depicted in Fig. 1, A and B, respectively. Tamoxifen treatment was associated with over 90% satellite cell depletion in diaphragm of adult and aged mice independent of hypoxia status (main effect for drug treatment at both ages, P < 0.0001, Fig. 1, C and D). Diaphragm satellite cell density was lower in aged mice than in young (main effect for age, P < 0.0001, Fig. 1, C and D). Red blood cell (RBC) concentration, hemoglobin concentration (HB), and hematocrit (HCT) were determined in blood of young mice to ensure the effects of hypoxia treatment. RBC, HB, and HCT were all higher in hypoxia-treated mice than in normoxia-treated mice (Supplemental Table S1; main effect for hypoxia, P < 0.001). Satellite cell depletion did not impact RBC, HB, or HCT (Supplemental Table S1; P = 0.776, P = 0.984, P = 0.879, respectively). Hypoxia did not significantly affect satellite cell density in adult or aged diaphragm (P = 0.103, Fig. 1, C and D). Myonuclear number was not different in diaphragm muscle with age, satellite cell depletion, or hypoxia (Fig. 1, E and F).
Figure 1.
Satellite cell depletion does not affect myonuclear number in young and old diaphragm with or without hypoxia. Representative images of vehicle-treated (A) and tamoxifen-treated (B) Pax7-DTA mouse diaphragm; scale bars represent 25 µm. Pax7+ cell count in vehicle- and tamoxifen-treated adult (C) and aged (D) diaphragm in normoxia and hypoxia. Myonuclei/100 µm in adult (E) and aged (F) diaphragm. *Main effect for tamoxifen treatment (P < 0.0001), #main effect for age (P < 0.0001). C and D: adult normoxia veh group n = 9, adult normoxia tam group n = 5, adult hypoxia veh group n = 8, adult hypoxia tam group n = 8, aged normoxia veh group n = 7, aged normoxia tam group n = 7, aged hypoxia veh group n = 7, aged hypoxia tam group n = 10. E and F: adult normoxia veh group n = 7, adult normoxia tam group n = 5, adult hypoxia veh group n = 9, adult hypoxia tam group n = 8, aged normoxia veh group n = 6, aged normoxia tam group n = 8, aged hypoxia veh group n = 8, aged hypoxia tam group n = 11. Differences in Pax7+ cell density and myonuclear cell density were tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Error bars represent SE. tam, tamoxifen; veh, vehicle.
CSA Is Lower with Age and Hypoxia, but Not Affected by Satellite Cell Depletion
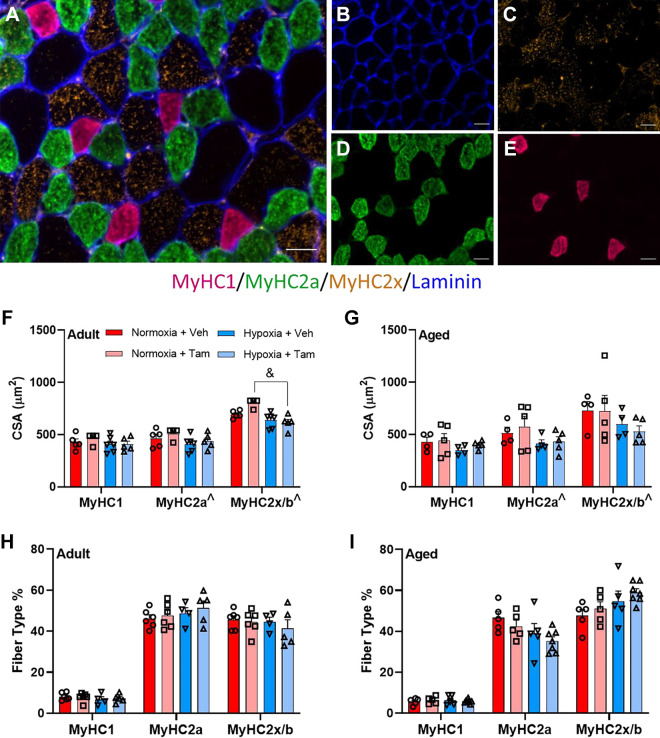
CSA, as measured using dystrophin staining (Fig. 2A) was lower in diaphragm muscle in aged mice (main effect, P < 0.001) and with hypoxia (main effect, P = 0.0004) (Fig. 2, B and C). A representative muscle section of MyHC staining is depicted in Fig. 3, A–E. Hypoxia was associated with lower CSA of MyHC2a and MyHC2x/b fibers in diaphragm in both vehicle- and tamoxifen-treated mice at both ages, (main effect, P = 0.0157 and P = 0.0069, respectively) (Fig. 3, F and G). Hypoxia led to lower MyHC2x/b CSA in satellite cell-depleted adult, but not aged mice (P < 0.001) (Fig. 3F). Fiber-type distribution was not affected by satellite cell depletion, age, or hypoxia (Fig. 3, H and I).
Figure 2.
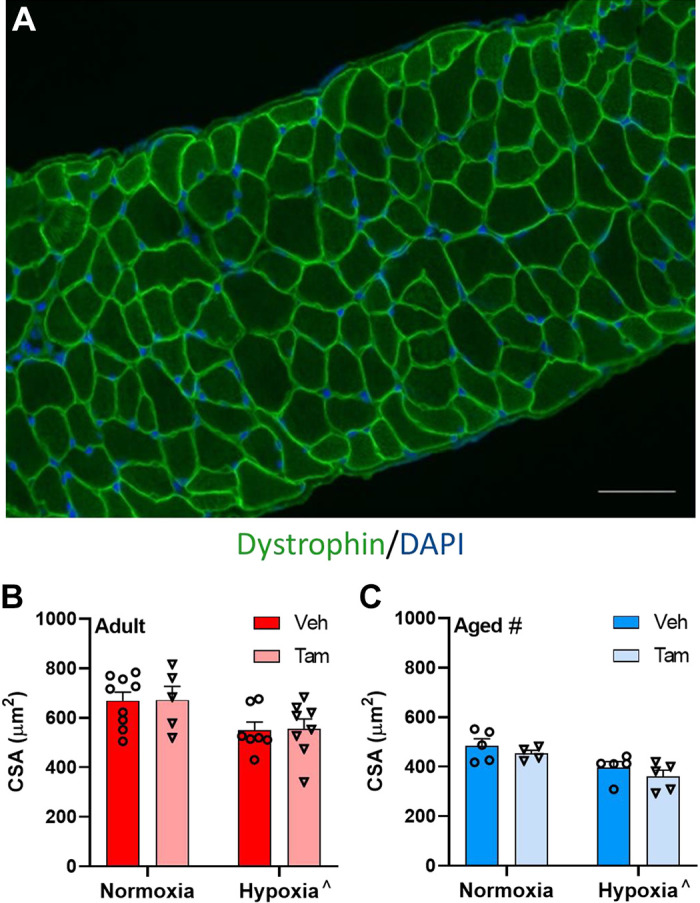
Satellite cell depletion does not affect cross-sectional area in diaphragm with or without hypoxia. Representative image of dystrophin antibody staining on mouse diaphragm; scale bar represents 150 µm (A). Pooled cross-sectional area of vehicle-treated and tamoxifen-treated adult (B) and aged (C) diaphragm. ^Main effect for hypoxia (P = 0.002); #main effect for age (P < 0.0071). Adult normoxia veh group n = 9, adult normoxia tam group n = 5, adult hypoxia veh group n = 7, adult hypoxia tam group n = 8, aged normoxia veh group n = 5, aged normoxia tam group n = 4, aged hypoxia veh group n = 5, aged hypoxia tam group n = 5. Differences in cross-sectional area were tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Error bars represent SE. tam, tamoxifen; veh, vehicle.
Figure 3.
Hypoxia, but not satellite cell depletion lowers MyHC2a and MyHC2x/b muscle fiber cross-sectional area with no impact on fiber-type distribution from hypoxia or satellite cell depletion. Representative images of fiber-type stain on diaphragm, merged (A), laminin (B), type IIx (C), type IIb (D), and type I (E); scale bars represent 25 µm. Fiber-type specific cross-sectional area of adult (F) and aged (G) mouse diaphragm. Fiber-type distribution of adult (H) and aged (I) diaphragm. ^Main effect for hypoxia in cross-sectional area of MyHC2a and MyHC2x/b fibers (P = 0.0157 and P = 0.0069, respectively). &Significant differences between groups, within fiber type. Adult normoxia veh group n = 5, adult normoxia tam group n = 6, adult hypoxia veh group n = 4, adult hypoxia tam group n = 5, aged normoxia veh group n = 5, aged normoxia tam group n = 5, aged hypoxia veh group n = 5, aged hypoxia tam group n = 7. Differences in fiber-type specific cross-sectional area and fiber-type distribution for MyHC1, MyHC2a, and MyHC2b/x were individually tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Error bars represent SE. MyHC, myosin heavy chain; tam, tamoxifen; veh, vehicle.
Diaphragm Extracellular Matrix Content Is Higher in Aged Independent of Satellite Cell Depletion or Hypoxia
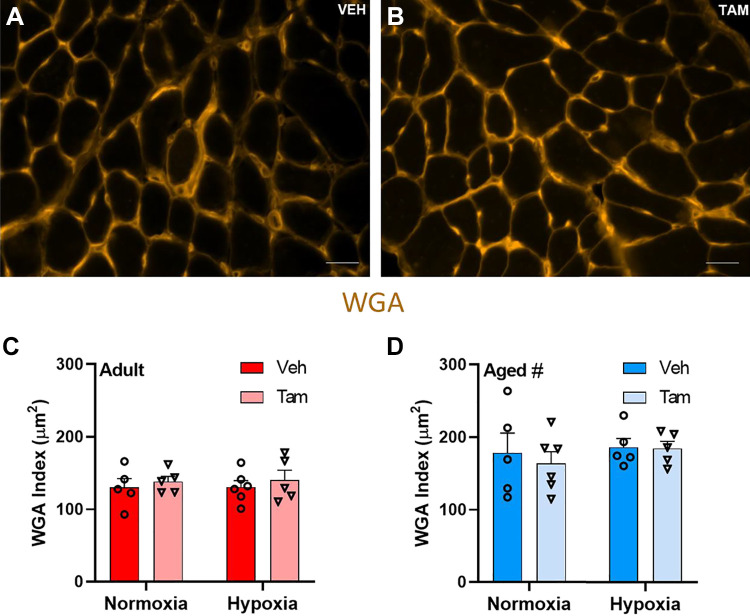
Extracellular matrix content as measured by WGA index (representative images of vehicle (Fig. 4A) and tamoxifen-treated (Fig. 4B) diaphragm was higher in aged than in adult mice (main effect, P = 0.0002). No differences were observed in diaphragm extracellular matrix content between tamoxifen-treated and vehicle-treated or between hypoxia- and normoxia-treated mice (Fig. 4, C and D).
Figure 4.
Extracellular matrix content in diaphragm is not different with satellite cell depletion and higher with age. Representative images of vehicle-treated (A) and tamoxifen-treated (B) Pax7-DTA mouse diaphragm stained for WGA; scale bars represent 25 µm. WGA index (µm2) for vehicle- and tamoxifen-treated adult (C) and aged (D) diaphragm for normoxia and hypoxia. #Main effect for age (P = 0.0002). Adult normoxia veh group n = 5, adult normoxia tam group n = 5, adult hypoxia veh group n = 6, adult hypoxia tam group n = 5, aged normoxia veh group n = 5, aged normoxia tam group n = 6, aged hypoxia veh group n = 5, aged hypoxia tam group n = 5. Differences in extracellular matrix content was tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Error bars represent SE. tam, tamoxifen; veh, vehicle; WGA, wheat germ agglutinin.
The Number of Pax3+ Cells is Higher with Satellite Cell Depletion and is Lower with Age, but Not Different with Hypoxia
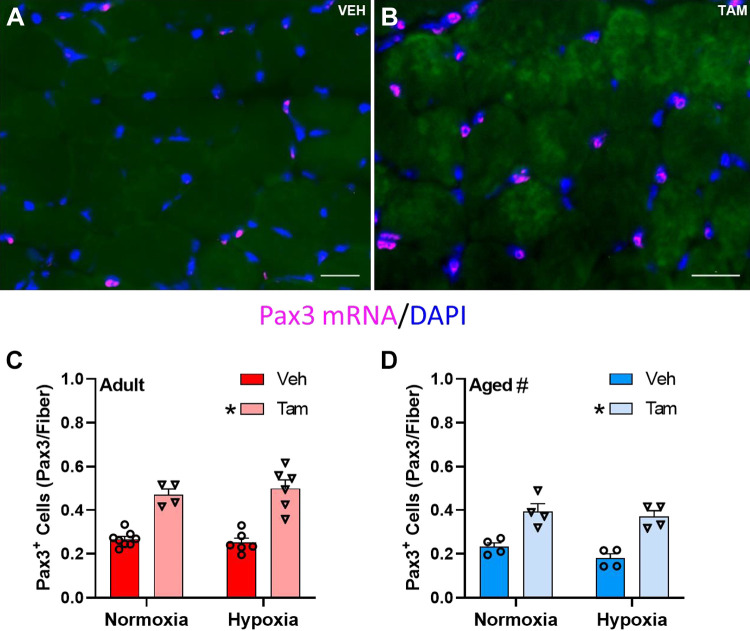
Figure 5, A and B, depicts representative images of Pax3 FISH in diaphragm of vehicle- and tamoxifen-treated mice, respectively. The number of Pax3+ cells was higher in satellite cell-depleted than in nondepleted diaphragm muscle of both adult and aged mice (main effect, P < 0.0001, Fig. 5, C and D). Pax3+ cells were lower in aged diaphragm (main effect, P = 0.0002, Fig. 5, C and D). Hypoxia did not affect Pax3+ cell number in diaphragm of adult or aged mice with or without satellite cell depletion.
Figure 5.
Pax3 mRNA expression in diaphragm is higher with tamoxifen treatment and lower with age. Representative images of vehicle-treated (A) and tamoxifen-treated (B) Pax7-DTA mouse diaphragm; scale bars represent 25 µm. Pax3 mRNA+ cell count in vehicle- and tamoxifen-treated adult (C) and aged (D) diaphragm in normoxia and hypoxia. *Main effect for tamoxifen treatment (P < 0.0001); #main effect for age (P = 0.0002). Adult normoxia veh group n = 8, adult normoxia tam group n = 4, adult hypoxia veh group n = 6, adult hypoxia tam group n = 6, aged normoxia veh group n = 4, aged normoxia tam group n = 4, aged hypoxia veh group n = 4, aged hypoxia tam group n = 4. Differences in Pax3+ cell density was tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Error bars represent SE. tam, tamoxifen; veh, vehicle.
In Vivo Diaphragm Function Was Altered by Hypoxia, but Not Satellite Cell Depletion
Aged mice had lower expiratory rate, inspiratory rate, and overall breath rate (breaths/min) than adult mice (Table 1 main effect of age, P = 0.0002, P = 0.0049, P < 0.0001, respectively). Breath depth and expiratory rate were greater with hypoxia treatment in adult (Table 1 main effect, P = 0.0411, P = 0.0414, respectively), but not aged mice. Inspiratory rate was higher with hypoxia treatment in aged mice (Table 1 main effect, P = 0.0161), but not adult.
Table 1.
In vivo breathing dynamics in aged and young adult vehicle- and tamoxifen-treated mice
| Adult |
||||
|---|---|---|---|---|
| Veh |
Tam |
|||
| Normoxic (n = 3) | Hypoxic (n = 4) | Normoxic (n = 3) | Hypoxic (n = 3) | |
| Depth, mm | 0.85 ± 0.05 | 1.00 ± 0.11^ | 0.93 ± 0.11 | 1.42 ± 2.82^ |
| Breath/min | 308.53 ± 21.87 | 342.78 ± 15.42 | 295.6 ± 21.87 | 286.85 ± 15.42 |
| Inspiration, mm/s | 9.44 ± 1.51 | 12.66 ± 1.91 | 12.68 ± 1.51 | 15.96 ± 1.91 |
| Expiration, mm/s | 9.02 ± 0.24 | 12.75 ± 1.83^ | 10.93 ± 0.85 | 15.71 ± 1.83^ |
| Aged |
||||
|---|---|---|---|---|
| Veh |
Tam |
|||
| Normoxic (n = 8) | Hypoxic (n = 3) | Normoxic (n = 3) | Hypoxic (n = 3) | |
| Depth, mm | 1.06 ± 0.07 | 1.20 ± 0.13 | 1.01 ± 0.09 | 1.25 ± 0.10 |
| Breath/min# | 231.26 ± 11.03 | 221.66 ± 48.51 | 199.57 ± 15.36 | 214.31 ± 14.74 |
| Inspiration, mm/s# | 9.75 ± 0.83 | 9.81 ± 0.65^ | 7.84 ± 0.58 | 12.72 ± 0.96^ |
| Expiration, mm/s# | 7.85 ± 0.85 | 6.84 ± 1.86 | 7.38 ± 1.09 | 8.48 ± 1.25 |
Data are shown as means ± SE. Significance: #main effect of age, ^main effect of hypoxia (P < 0.05 for main effects). Differences in in vivo breathing dynamics were individually tested using two-way ANOVA with Šídák’s multiple comparison correction. Age effects were detected using three-way ANOVA. Tam, satellite cell ablated; Veh, vehicle treated.
DISCUSSION
The goal of this study was to determine the impact of satellite cell depletion on diaphragm adaptations to hypoxia in adult and aged mice. We hypothesized that satellite cell depletion negatively impacts indices of diaphragm muscle morphology and function showing greater impairments in aged than adult mice. Contrary to our hypothesis, satellite cell depletion did not affect diaphragm muscle morphology or function. Advanced age is associated with smaller muscle fiber cross-sectional area and higher ECM content (35) and hypoxia is well known to induce a decrease in muscle fiber size (36–41). These age- and hypoxia-associated adaptations were confirmed in our study regardless of the presence of satellite cells.
We previously showed that satellite cell depletion did not affect adaptations to voluntary wheel running in the diaphragm of adult and aged mice (24). This result was somewhat unexpected, because it has been shown that satellite cells fuse into diaphragm muscle at a much higher rate than hindlimb muscles (21, 22) and therefore diaphragm was expected to be compromised when satellite cells are ablated, particularly in the aged. It was suggested that voluntary wheel running may not have been a robust enough stimulus to elicit differences in diaphragm muscle adaptation with satellite cell depletion (25). Therefore, we aimed to challenge the diaphragm using hypoxia, a stimulus known to chronically increase ventilatory frequency (26, 27). In addition, chronic environmental hypoxia exposure has a marked influence on satellite cell behavior; miR-1 and miR-206, which typically promote terminal differentiation of satellite cells (28, 42), are suppressed by hypoxia, whereas MyoD degradation (30) and Notch signaling (28, 31) are enhanced. Together, these hypoxia-mediated effects are associated with decreased satellite cell differentiation capacity and enhanced cell self-renewal. If these stem cell adaptations are important for maintaining muscle integrity during hypoxia, the diaphragm should have been compromised during satellite cell ablation, which was not in the current study. Our results, therefore, indicate that hypoxia-induced changes to satellite cells are not necessary for adaptations in diaphragm muscle of adult or aged mice.
Satellite cells have the capacity to influence skeletal muscle ECM by regulating the expression of ECM-associated genes in fibro-adipogenic progenitor cells and fibroblasts during hypertrophy (43–46). The role of satellite cells in regulating ECM turnover of nonhypertrophying muscle is less clear; we showed that plantaris in adult and diaphragm muscle in adult and aged mice did not accumulate ECM when satellite cells are depleted (24, 47). Our current study shows similar results in response to satellite cell ablation, although ECM was elevated in aged diaphragm muscle as has been shown previously for hindlimb muscles (48). Thus, our results indicate that the absence of satellite cells does not exacerbate the accumulation of ECM with age in diaphragm muscle. Pathological ECM expansion has more recently become appreciated as a mechanism by which satellite cell activity is impaired with some evidence suggesting satellite cells transition from a myogenic to fibrogenic phenotype in models of fibrosis (49, 50). Although satellite cell depletion did not affect ECM content, it is conceivable that the lower satellite cell density observed in aged mouse diaphragm concomitant with the increase in ECM content in aged mice was due to a fibrogenic conversion of other stem cells, promoting fibrosis.
It needs to be noted that in the current study we used males, whereas in the previous study on the effect of running with satellite cell depletion in diaphragm, females were used (24). Females have lower satellite cell density than males, potentially explained by the female-specific estrogen sensitivity of satellite cells (51–53) and therefore it was expected that males might have a more severe response to an adaptation when satellite cells are depleted. This was however not the case in the current study as the lack of satellite cells did not affect the adaptation to hypoxia. In addition, we previously observed in females a greater proportion of slow, oxidative fibers in diaphragm muscle following 8 wk of voluntary wheel running (24), whereas in the current study, we did not observe any fiber-type distribution differences in males with 4 wk of hypoxia treatment. It is possible that the length of treatment (8 wk vs. 4 wk) was responsible for the difference in response between males and females or the strength of the stimulus (running vs. hypoxia). Lastly, diaphragm ECM content was not different in adult compared with aged female mice (24); in the current study, male mice demonstrated higher ECM content in the aged, suggesting that females are more resistant to ECM accumulation in diaphragm muscle with age. In contrast, TGF-β, a key fibrogenic gene, is more highly expressed in females than in males, however, this study did not measure ECM content and was not performed in diaphragm muscle (35). It is possible that females have greater ECM turnover than males, potentially explaining the greater TGF-β expression in females. Therefore, there are sex-related differences in the diaphragm with aging, but for both males and females, the adaptation of diaphragm muscle to hypoxia and running, respectively, does not require satellite cells.
Satellite cell depletion was associated with a significantly higher number of Pax3 mRNA+ (herein referred to as Pax3+) cells in the diaphragm, consistent with our previous study (24). Pax3 is typically coexpressed with Pax7 to give rise to satellite cells and direct a myogenic lineage in developing muscle fibers (54, 55). The increase in Pax3-expressing cells in diaphragm muscle fibers independent of Pax7 expression suggests that a compensatory program exists to overcome satellite cell depletion in diaphragm muscle. In developing muscle, Pax3 expression without Pax7 expression typically results in apoptosis (56), however, Pax3 promotes myogenic cell viability in adult muscle (57). As Pax7+ cells were depleted at 4 mo of age in the mice, we suggest that the Pax3+ cells are of an adult myogenic lineage and not of a developmental myogenic lineage. Furthermore, we show that vehicle, normoxia-treated adult mice have nearly 10-fold higher Pax3+ cells than Pax7+ cells, but the precise role of Pax3+ cells in promoting diaphragm adaptations is unknown. It is conceivable that Pax3+ cells are contributing to muscle adaptations that do not require fusion with myofibers and for which satellite cells would typically be responsible. ECM regulation is a key nonfusion process of satellite cells (45) and Pax3+ cells may be taking over this role in satellite cell-depleted diaphragm muscle, potentially explaining why we did not observe changes in diaphragm ECM content with satellite cell depletion. In addition, similar to the age-associated decline in Pax7-expressing cells, Pax3-expressing cells were lower in aged diaphragm than in adult in both satellite cell-depleted and replete diaphragm. This suggests the Pax3-dependent compensatory mechanism for a severely declining satellite cell pool is impaired with aging. The increase in Pax3-expressing cells in satellite cell-depleted diaphragm found in our study suggests diaphragm muscle fibers have an intrinsic compensatory mechanism to overcome satellite cell depletion and possibly compensate for the loss of satellite cells.
Despite our efforts to remain rigorous in our methods, this study contains limitations to consider when interpreting the data reported here. Hypoxia-treated mice were exposed to normoxia for a negligible amount of time during cage changes; a total of 15 min during the entire 4-wk hypoxia treatment. In addition to our respiratory function measures, we used a singular 4-wk endpoint to validate our hypoxia treatment by hemodynamic response, but this does not guarantee that the mice were in a steady state for hypoxia. Pax3 expression was only measured by FISH, a method to identify Pax3 mRNA and not protein. Lastly, respiratory function was measured via ultrasonography, as we have previously reported with similar findings (24), but other less stressful in vivo measures, such as plethysmography would be better assessments in future studies.
In summary, we show here that satellite cell depletion does not impact the adaptations to hypoxia in adult or old diaphragm muscle with respect to CSA, fiber-type distribution, ECM content, or myonuclear number. However, the higher number of Pax3-expressing cells in diaphragm muscle with satellite cell depletion potentially reveals a compensatory mechanism for the role of satellite cells in diaphragm muscle and needs to be further investigated.
SUPPLEMENTAL DATA
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20212064.v1.
GRANTS
This project was funded by National Institutes of Health-National Institute on Aging AG043721.
DISCLOSURES
E. E. Dupont-Versteegden is an editor of Journal of Applied Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.C. and E.E.D-V. conceived and designed research; A.L.C. and E.E.D-V. performed experiments; N.T.T. and E.E.D-V. analyzed data; N.T.T. and E.E.D-V. interpreted results of experiments; N.T.T. and E.E.D-V. prepared figures; N.T.T., C.S.F., and E.E.D-V. drafted manuscript; N.T.T., A.L.C., C.S.F., and E.E.D-V. edited and revised manuscript; N.T.T., A.L.C., C.S.F., and E.E.D-V. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract was created using BioRender.com and published with permission.
REFERENCES
- 1. Kelley RC, McDonagh B, Ferreira LF. Advanced aging causes diaphragm functional abnormalities, global proteome remodeling, and loss of mitochondrial cysteine redox flexibility in mice. Experimental Gerontology 103: 69–79, 2018. doi: 10.1016/j.exger.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Criswell DS, Powers SK, Herb RA, Dodd SL. Mechanism of specific force deficit in the senescent rat diaphragm. Respiration Physiology 107: 149–155, 1997. doi: 10.1016/S0034-5687(96)02509-1. [DOI] [PubMed] [Google Scholar]
- 3. Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest 124: 2302–2308, 2003. doi: 10.1378/chest.124.6.2302. [DOI] [PubMed] [Google Scholar]
- 4. Fogarty MJ, Marin Mathieu N, Mantilla CB, Sieck GC. Aging reduces succinate dehydrogenase activity in rat type IIx/IIb diaphragm muscle fibers. J Appl Physiol (1985) 128: 70–77, 2020. doi: 10.1152/japplphysiol.00644.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med 150: 174–178, 1994. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- 6. Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polkey MI, Harris ML, Hughes PD, Hamnegärd C, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med 155: 1560–1564, 1997. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- 8. Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med 152: 677–682, 1995. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- 9. Vang P, Vasdev A, Zhan W-Z, Gransee HM, Sieck GC, Mantilla CB. Diaphragm muscle sarcopenia into very old age in mice. Physiol Rep 8: e14305, 2020. doi: 10.14814/phy2.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lepper C, Partridge TA, Fan C-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656, 2011. [Erratum in Development 138: 4333, 2011]. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 12. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackey AL, Kjaer M. The breaking and making of healthy adult human skeletal muscle in vivo. Skeletal Muscle 7: 24, 2017. doi: 10.1186/s13395-017-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am J Physiol Cell Physiol 303: C854–C861, 2012. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mañas-García L, Guitart M, Duran X, Barreiro E. Satellite cells and markers of muscle regeneration during unloading and reloading: effects of treatment with resveratrol and curcumin. Nutrients 12: 1870, 2020. doi: 10.3390/nu12061870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell PO, Pavlath GK. A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol 281: C1706–C1715, 2001. doi: 10.1152/ajpcell.2001.281.5.C1706. [DOI] [PubMed] [Google Scholar]
- 17. Finnerty CC, McKenna CF, Cambias LA, Brightwell CR, Prasai A, Wang Y, El Ayadi A, Herndon DN, Suman OE, Fry CS. Inducible satellite cell depletion attenuates skeletal muscle regrowth following a scald-burn injury. J Physiol 595: 6687–6701, 2017. doi: 10.1113/JP274841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Englund DA, Figueiredo VC, Dungan CM, Murach KA, Peck BD, Petrosino JM, Brightwell CR, Dupont AM, Neal AC, Fry CS, Accornero F, McCarthy JJ, Peterson CA. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function (Oxf) 2: zqaa033, 2021. doi: 10.1093/function/zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA, Dungan CM. Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. J Cachexia Sarcopenia Muscle 11: 1705–1722, 2020. doi: 10.1002/jcsm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6: 7087, 2015. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pawlikowski B, Pulliam C, Betta ND, Kardon G, Olwin BB. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5: 42, 2015. doi: 10.1186/s13395-015-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21: 76–80, 2015. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murach KA, Confides AL, Ho A, Jackson JR, Ghazala LS, Peterson CA, Dupont-Versteegden EE. Depletion of Pax7+ satellite cells does not affect diaphragm adaptations to running in young or aged mice. J Physiol 595: 6299–6311, 2017. doi: 10.1113/JP274611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Lisio M, Farup J. The role of satellite cells in activity-induced adaptations: breathing new life into the debate. J Physiol 595: 6225–6226, 2017. doi: 10.1113/JP274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ivy CM, Scott GR. Ventilatory acclimatization to hypoxia in mice: methodological considerations. Respir Physiol Neurobiol 235: 95–103, 2017. doi: 10.1016/j.resp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 27. Cramer NP, Xu X, Christensen C, Bierman A, Tankersley CG, Galdzicki Z. Strain variation in the adaptation of C57Bl6 and BALBc mice to chronic hypobaric hypoxia. Physiol Behav 143: 158–165, 2015. doi: 10.1016/j.physbeh.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 28. Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, Kuang S. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development 139: 2857–2865, 2012. doi: 10.1242/dev.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elashry MI, Kinde M, Klymiuk MC, Eldaey A, Wenisch S, Arnhold S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res Ther 13: 56, 2022. doi: 10.1186/s13287-022-02730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J Biol Chem 279: 16332–16338, 2004. doi: 10.1074/jbc.M313931200. [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 9: 617–628, 2005. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 32. Gamboa JL, Andrade FH. Mitochondrial content and distribution changes specific to mouse diaphragm after chronic normobaric hypoxia. Am J Physiol Regul Integr Comp Physiol 298: R575–R583, 2010. doi: 10.1152/ajpregu.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zuo L, Roberts WJ, Evans KD. Diagnostic ultrasound imaging of mouse diaphragm function. J Vis Exp 21: 51290, 2014. doi: 10.3791/51290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wen Y, Murach KA, Vechetti IJ Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol (1985) 124: 40–51, 2018. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parker L, Caldow MK, Watts R, Levinger P, Cameron-Smith D, Levinger I. Age and sex differences in human skeletal muscle fibrosis markers and transforming growth factor-β signaling. Eur J Appl Physiol 117: 1463–1472, 2017. doi: 10.1007/s00421-017-3639-4. [DOI] [PubMed] [Google Scholar]
- 36. Chaudhary P, Suryakumar G, Prasad R, Singh SN, Ali S, Ilavazhagan G. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin–proteasome pathway and calpains. Mol Cell Biochem 364: 101–113, 2012. doi: 10.1007/s11010-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 37. Ferretti G, Hauser H, Di Prampero P. Maximal muscular power before and after exposure to chronic hypoxia. Int J Sports Med 11: S31–S34, 1990. doi: 10.1055/s-2007-1024851. [DOI] [PubMed] [Google Scholar]
- 38. Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar S, Cerretelli PII. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med 11: S3–S9, 1990. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- 39. Raguso CA, Guinot SL, Janssens J-P, Kayser B, Pichard C. Chronic hypoxia: common traits between chronic obstructive pulmonary disease and altitude. Curr Opin Clin Nutr Metab Care 7: 411–417, 2004. doi: 10.1097/01.mco.0000134372.78438.09. [DOI] [PubMed] [Google Scholar]
- 40. Rose MS, Houston CS, Fulco CS, Coates G, Sutton JR, Cymerman A. Operation Everest. II: Nutrition and body composition. J Appl Physiol (1985) 65: 2545–2551, 1988. doi: 10.1152/jappl.1988.65.6.2545. [DOI] [PubMed] [Google Scholar]
- 41. de Theije CC, Langen RCJ, Lamers WH, Schols AMWJ, Köhler SE. Distinct responses of protein turnover regulatory pathways in hypoxia-and semistarvation-induced muscle atrophy. Am J Physiol Lung Cell Mol Physiol 305: L82–L91, 2013. doi: 10.1152/ajplung.00354.2012. [DOI] [PubMed] [Google Scholar]
- 42. Chen J-F, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang D-Z. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol 190: 867–879, 2010. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont‐Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber micro environment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murach KA, Vechetti IJ Jr, Van Pelt DW, Crow SE, Dungan CM, Figueiredo VC, Kosmac K, Fu X, Richards CI, Fry CS, McCarthy JJ, Peterson CA. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function (Oxf) 1: zqaa009, 2020. doi: 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murach KA, Peck BD, Policastro RA, Vechetti IJ, Van Pelt DW, Dungan CM, Denes LT, Fu X, Brightwell CR, Zentner GE, Dupont-Versteegden EE, Richards CI, Smith JJ, Fry CS, McCarthy JJ, Peterson CA. Early satellite cell communication creates a permissive environment for long-term muscle growth. Iscience 24: 102372, 2021. doi: 10.1016/j.isci.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jackson JR, Kirby TJ, Fry CS, Cooper RL, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Reduced voluntary running performance is associated with impaired coordination as a result of muscle satellite cell depletion in adult mice. Skelet Muscle 5: 41, 2015. doi: 10.1186/s13395-015-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lacraz G, Rouleau A-J, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One 10: e0136217, 2015. [Erratum in PLoS One 11: e0167661, 2016]. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stearns-Reider KM, D'Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16: 518–528, 2017. doi: 10.1111/acel.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol 293: C661–C669, 2007. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 51. Neal A, Boldrin L, Morgan J. The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration. PLoS One 7: e37950, 2012. doi: 10.1371/journal.pone.0037950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi H-K, Laakkonen EK, Sipilä S, Kovanen V, Spangenburg EE, Kyba M, Lowe DA. Estrogen regulates the satellite cell compartment in females. Cell Rep 28: 368–381.e6, 2019. doi: 10.1016/j.celrep.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seko D, Fujita R, Kitajima Y, Nakamura K, Imai Y, Ono Y. Estrogen receptor β controls muscle growth and regeneration in young female mice. Stem Cell Reports 15: 577–586, 2020. doi: 10.1016/j.stemcr.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435: 948–953, 2005. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 55. Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomès D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 19: 1426–1431, 2005. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Boutet SC, Disatnik M-H, Chan LS, Iori K, Rando TA. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell 130: 349–362, 2007. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 57. Der Vartanian A, Quétin M, Michineau S, Auradé F, Hayashi S, Dubois C, Rocancourt D, Drayton-Libotte B, Szegedi A, Buckingham M, Conway SJ, Gervais M, Relaix F. PAX3 confers functional heterogeneity in skeletal muscle stem cell responses to environmental Stress. Cell Stem Cell 24: 958–973.e9, 2019. doi: 10.1016/j.stem.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20212064.v1.