Figure 1.
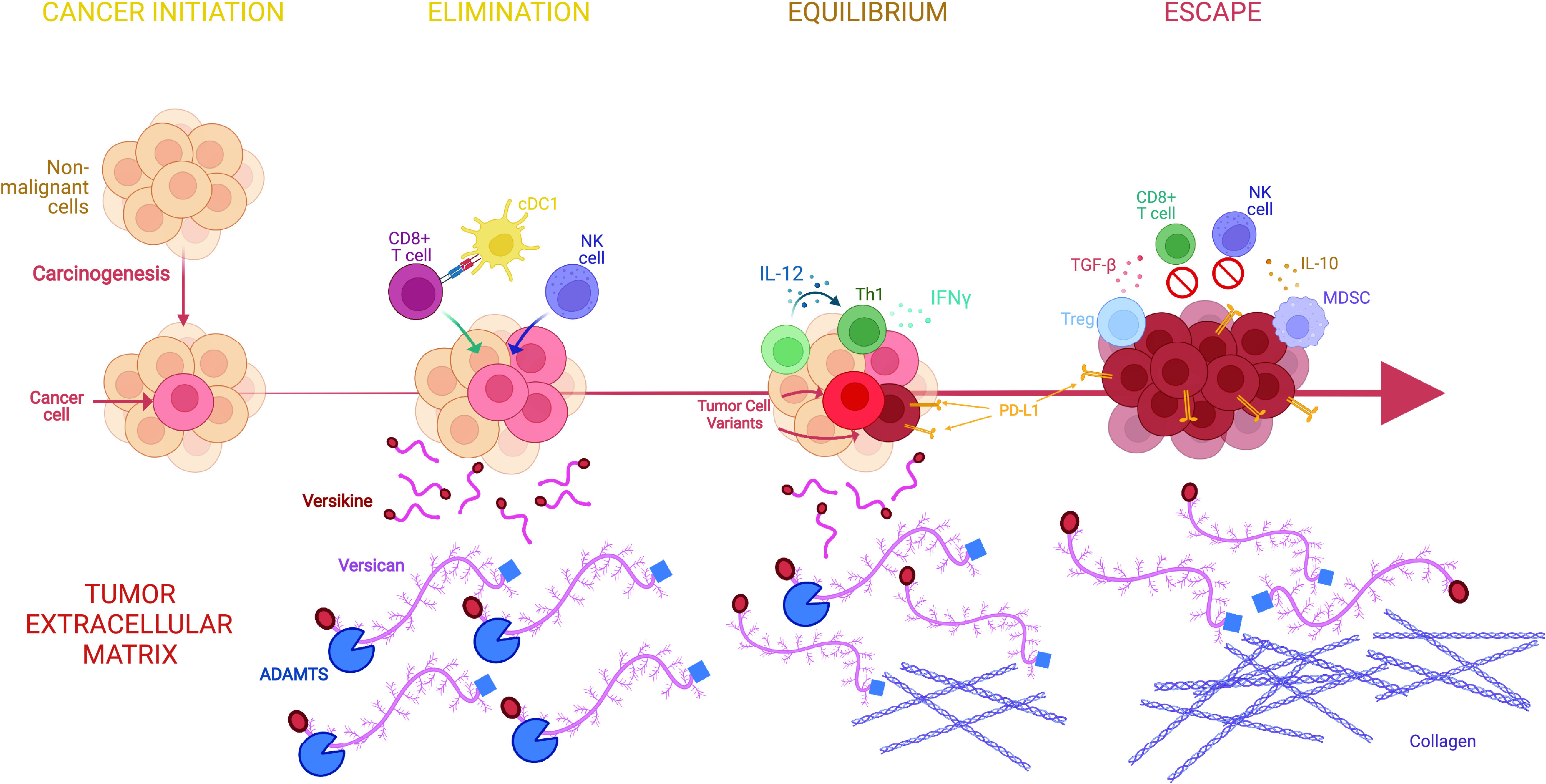
Matrix proteoglycans in cancer evolution and immunoediting. Cancers are “wounds that do not heal.” Immunoediting refers to the distinct stages of interfacing between the host immune system and the developing cancer. There are three stages: elimination, equilibrium, and escape (see text for details). Extracellular matrix remodeling regulates key signaling pathways at each step. The case is illustrated by versican (VCAN), a versatile large matrix proteoglycan that possesses diverse context-specific immunoregulatory or immunostimulatory roles, depending on its abundance, isoform structure, and proteolysis status (10). We propose the following model based on work by our group and others (11–13): during elimination, robust VCAN proteolysis releases the immunostimulatory fragment (matrikine), versikine, that regulates stimulatory dendritic cells (conventional type 1 dendritic cells, cDC1) promoting immune destruction of the tumor through enhanced neoantigen recognition. In equilibrium, a precarious balance is established between immunostimulatory proteolyzed VCAN products and parental, nonproteolyzed immunoregulatory VCAN that allows the propagation of tumors with active, but progressively ineffectual, immune surveillance. In escape, progressive fibrosis and immunosuppression [e.g., through TGF-β (14)] attenuates VCAN proteolysis and results in the unbalanced immunoregulatory activity of nonproteolyzed VCAN. In these latter stages, immunosuppressive signals from receptors recognizing collagen may further promote immune exclusion (15, 16). Created with BioRender.com.
