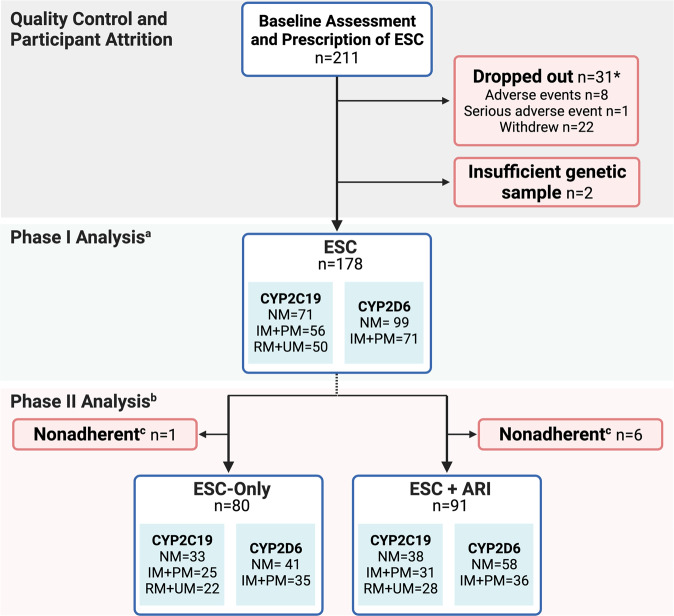
Fig. 1. Process of research design.
a During Phase I (Weeks 0–8), all participants received open-label ESC monotherapy (10–20 mg/d, flexible dosage). On Week 8, participants were classified as responders (≥50% decrease from baseline in MADRS scores) or non-responders (<50% decrease from baseline in MADRS scores). b During Phase II (Weeks 8–16), responders continued ESC monotherapy, while non-responders received ARI (2–10 mg/d, flexible dosage) augmentation in addition to ESC. c Adherence to the study medication was confirmed in participants based on the detection of ESC in serum at Weeks 2, 10, and 16, and the detection of ARI in serum at Weeks 10 and 16 for the ESC + ARI treatment arm. All participants were adherent to treatment during Phase I based on serum levels of the drug at Week 2. During Phase II, seven participants were non-adherent determined by a lack of treatment medication detected in serum at both Weeks 10 and 16, therefore they were not included in the Phase II analyses. ARI aripiprazole, ESC escitalopram, IM intermediate metabolizer, MADRS Montgomery–Åsberg Depression Rating Scale, NM normal metabolizer, PM poor metabolizer, RM rapid metabolizer, UM ultra-rapid metabolizer. *For details on the CAN-BIND 1 study protocol and a description of the sample, see ref. [27].