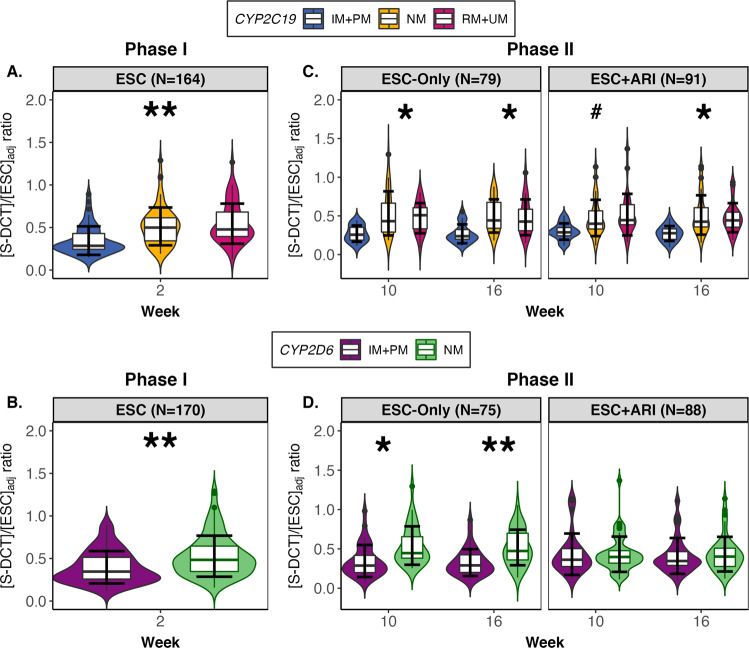
Fig. 5. Dose-adjusted serum S-DCT/ESCadj ratio for Phase I and II by CYP2C19 and CYP2D6 metabolizer groups.
During Phase I, A CYP2C19 and B CYP2D6 IM + PMs showed lower mean S-DCTad/ESCadj ratio relative to NMs. Likewise, during Phase II, in the ESC-Only and ESC + ARI treatment arms, C CYP2C19 IM + PMs compared to NMs had lower mean S-DCTad/ESCadj ratio in serum. D For CYP2D6, IM + PMs displayed lower S-DCTad/ESCadj ratio in ESC-Only, whereas S-DCTad/ESCadj ratio was not associated with CYP2D6 metabolizer group in the ESC + ARI treatment arm. All linear regression analyses were adjusted for age, ancestry, sex, site, time since last dose, CYP2C19 and CYP2D6 metabolizer groups. Error bars represent standard error. ARI aripiprazole, ESC escitalopram, IM intermediate metabolizer, NM normal metabolizer, PM poor metabolizer, RM rapid metabolizer, UM ultra-rapid metabolizer. *q < 0.05; **q < 0.01; ***q < 0.001.