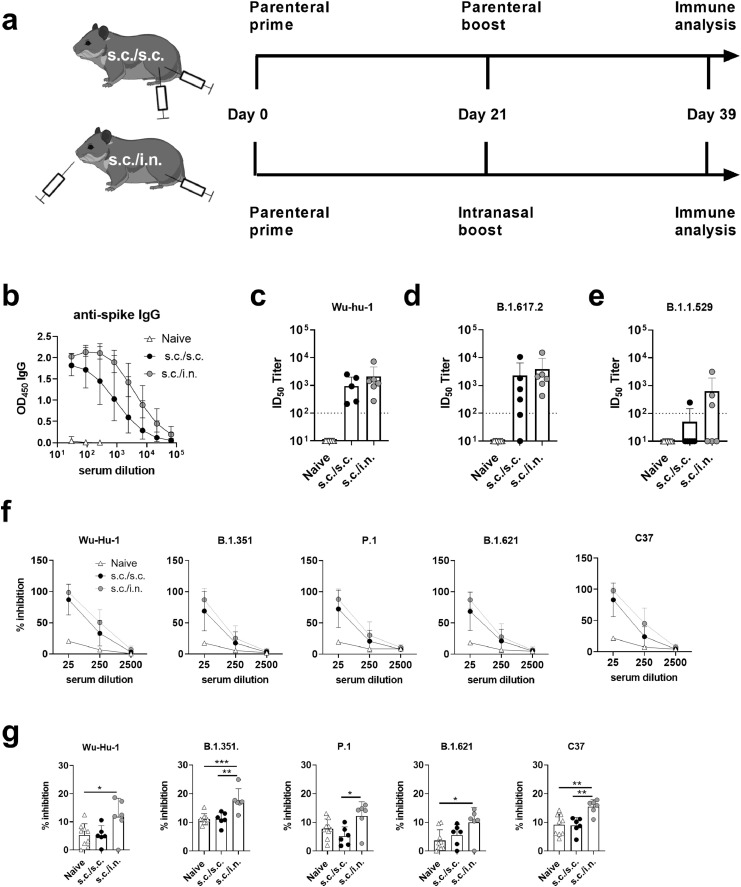
Figure 2.
Syrian Hamsters were immunized with two doses of spike HexaPro trimer protein formulated in cationic liposomes (CAF®01). The vaccine was either administered as a subcutaneous two dose regimen (s.c./s.c.) or as subcutaneous priming followed by intranasal boosting (s.c./i.n). a) Experimental setup. b) Serum IgG antibody responses against spike protein (mean+95% CI). Serum neutralization of SARS-CoV-2 was tested in a culture derived SARS-CoV-2 assay against c) the homologous Wu-hu-1 strain and d) the delta variant (B.1.617.2). A SARS-CoV-2 spike neutralizing monoclonal antibody (40592-MM57) was used as positive control at 1/800 dilution, which gave an average of 81% neutralization for the homologous Wu-Hu-1 variant and 96% for the B.1.617.2 variant. e) Neutralization of the omicron variant (B.1.1.529). Plasma from a COVID-19 vaccinated individual (1/80 dilution) was used as positive control giving 95% neutralization. The stippled line indicates neutralization below the limit of detection and is plotted as ID50=100. f) ACE2 competition assay measuring serum antibodies towards the Wu-Hu-1, B.1.351, P.1, B.1.621 and C37 strains. g) ACE2 competition assay measuring nasal washes antibodies towards the Wu-Hu-1, B.1.351, P.1, B.1.621 and C37 strains. Bars indicate mean+SD. Statistically significant differences are indicated by *, ** or *** (one-way ANOVA, comparing the mean of each column with each other column, p<0.05, 0.01 or 0.001, respectively). There was no statistically significance among groups if not otherwise indicated. Figures represent n= nine unvaccinated (naïve) and six vaccinated hamsters per group. Created with BioRender.com.