Case Presentation
A 45-year-old man sought treatment at the ED during the third wave of the COVID-19 pandemic with a month-long history of fatigue, cough, myalgia, and hand stiffness. He did not report dyspnea. He had no past medical history and previously was fit and active, working as a farmer. He was a lifelong nonsmoker and had no family history of lung disease.
Physical Examination Findings
On physical examination, his peripheral oxygen saturation was 96% on room air, respiratory rate was 18 breaths/min, and he was febrile with a temperature of 38.7 °C (101.6 °F). He was hemodynamically stable with a BP of 129 mm Hg/82 mm Hg and heart rate of 80 beats/min. No evidence was found of finger clubbing, synovitis, or muscle weakness. On auscultation of the chest, reduced air entry at the lung bases was observed.
Diagnostic Studies
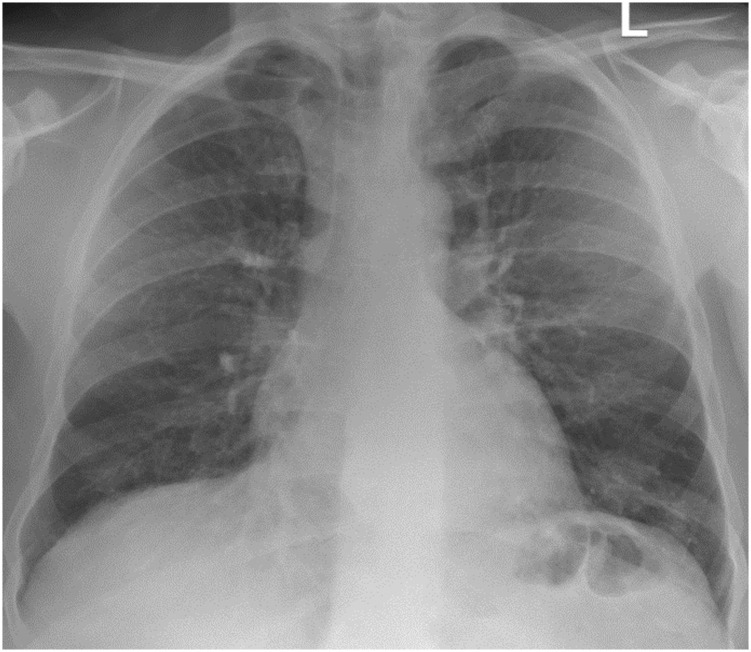
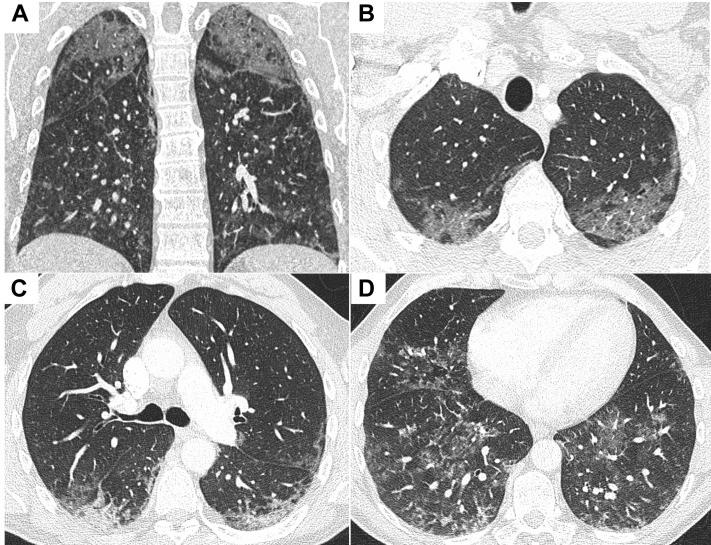
Initial blood testing revealed a WBC count of 7.6 × 109/L, and C-reactive protein level was elevated at 50 mg/L (normal range, 0-5.0 mg/L). Renal profile findings were within normal limits. N-terminal pro-brain natriuretic peptide level was 365 pg/mL (normal range, 0-121 pg/mL). Bloods culture and urinary Legionella and pneumococcal antigen findings were negative. Sputum culture grew Candida species. SARS-CoV-2 polymerase chain reaction results were negative on three separate occasions. Chest radiography revealed diffuse infiltrates throughout both lungs (Fig 1 ), and CT scan pulmonary angiography revealed diffuse bilateral ground-glass changes with reticulonodular opacifications in the lower lobes (Fig 2 ). Bronchoscopy and BAL cell differential demonstrated 75% macrophages and 11% lymphocytes. Microscopy and culture results for bacteria, mycobacteria, and fungi were negative on BAL.
Figure 1.
Posterior anterior chest radiograph demonstrating diffuse alveolar infiltrates bilaterally.
Figure 2.
A-D, CT pulmonary angiograms demonstrating diffuse bilateral ground-glass change with reticular nodular opacities opacification in the lower lobes: coronal image (A) and axial slices from lung apices to bases (B-D).
Empirical broad-spectrum antimicrobials were commenced and corticosteroids were added later to cover for hypersensitivity pneumonitis, given his farming background. This was added after the BAL was performed, while results were pending. Despite this, he demonstrated progressive hypoxemia, necessitating invasive mechanical ventilation on day 8 of admission. The rapidly progressive nature of this lung disease in the absence of identifiable infection raised suspicion for an inflammatory cause.
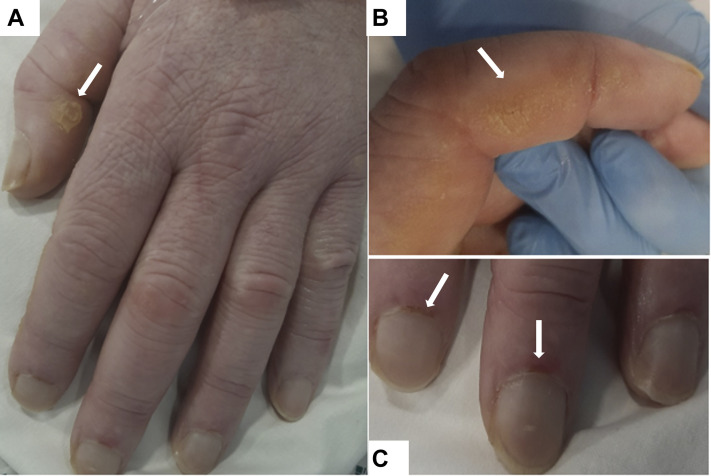
Autoantibody screening revealed positive speckled antinuclear antibody findings with a titer of 1:200, and anti-Ro antibodies were elevated at 131 U/mL (normal range, 0.0-6.9 U/mL). Antineutrophil cytoplasmic antibody, C3/C4, and immunoglobulin levels were normal. Creatine kinase level was elevated mildly at 686 U/L (normal range, 25-200 U/L) on admission, but normalized during his stay. He had no prior history of sicca or photosensitivity and no other features suggestive of systemic lupus erythematosus or Sjögren’s syndrome. Re-examination of his skin and joints identified subtle changes on his hands, with erythematous lesions over his interphalangeal joints, periungual erythema, and dry fissured skin suggestive of mechanic’s hands (Fig 3 ).
Figure 3.
A-C, Photographs showing hyperkeratosis and skin cracking (A, B) and periungual erythema and dilated nail-fold capillaries (C).
What is the diagnosis?
Diagnosis: Interstitial lung disease associated with anti-melanoma differentiation-associated protein 5 (MDA5) dermatomyositis
Discussion
Dermatomyositis is a heterogeneous disease that has many subtypes with varied clinical presentations and complications. The subtypes are identified by the presence of a myositis-specific antibody as well as their clinical presentation. Anti-MDA5 dermatomyositis is a recently recognized subtype that often presents with rapidly progressive interstitial lung disease. Without early recognition and aggressive immunosuppressive therapy, anti-MDA5 dermatomyositis has a poor prognosis and high mortality rate.
The anti-MDA5 antibody was identified first in 2005 in a Japanese cohort and initially was referred to as clinically amyopathic dermatomyositis 140 antibody. It was identified only in patients with clinically amyopathic dermatomyositis, encompassing those with amyopathic disease and hypomyopathic disease (patients with subclinical evidence of myositis). In this cohort, the presence of the clinically amyopathic dermatomyositis 140 antibody was associated with significantly more rapidly progressive interstitial lung disease compared with that in patients without the antibody. This antibody targets MDA-5; therefore, it is referred to as anti-MDA5. MDA5 is a protein involved in the innate immune system. It recognizes double-stranded RNA viruses, inducing a type 1 interferon response on recognition. Anti-MDA5 antibodies are dermatomyositis specific and help to differentiate from other connective tissue disorders or inflammatory myopathies, specifically antisynthetase syndrome, where the pathogenic antibody is directed against a transfer RNA synthetase (Table 1 ). Titers of anti-MDA5 antibody can correlate with disease activity and can be used to monitor response to treatment.
Table 1.
Clinical Features of Myositis-Related Interstitial Lung Diseases
| Variable | Anti-MDA-5 DM | Anti-synthetase Syndrome | Other Myositis-Related ILD |
|---|---|---|---|
| Myositis-specific antibodies | Anti-MDA-5 | Anti Jo-1, anti-PL7, anti-PL12, anti-OJ, anti-EJ, anti-KS, anti-Ha, and anti-Zo | Anti-PM-Scl, anti-R052, and anti-Ku |
| Muscle disease | Typically amyopathic or hypomyopathic | Myositis in 50%-90%; typical presentation with proximal muscle weakness | Myositis in 37%-49%; mixed pattern including proximal myopathy and myalgia |
| Skin disease | Ulceration and palmar papules specific to MDA-5; other DM skin manifestations: mechanic’s hands, heliotrope rash, Gottron’s papules, shawl sign | Mechanic’s hands, heliotrope rash, Gottron’s papules, shawl sign | Mechanic’s hands, subcutaneous edema, swollen hands, calcinosis, sclerodactyly, telangiectasia, malar rash, photosensitivity |
| ILD | Typically rapidly progressive | Typically chronic or subacute | Mixed |
| Frequency of ILD | 50%-90% | 66%-90% | 25%-61% |
| Other clinical features | Arthritis (60%), fever (60%) | Arthritis (60%), fever (25%), Raynaud’s phenomenon | Arthritis (77%-94%), Raynaud’s phenomenon (53%-78%) |
DM = dermatomyositis; ILD = interstitial lung disease; MDA-5 = melanoma differentiation-associated protein 5.
Anti-MDA5 dermatomyositis accounts for 20% to 40% of patients with dermatomyositis in Japan and 7% to 10% of patients with dermatomyositis in Europe. The exact pathogenesis of the disease is unknown; it likely is triggered by infectious or environmental factors in a genetically susceptible individual.
Anti-MDA5 dermatomyositis is associated with rapidly progressive interstitial lung disease and cutaneous disease, with little to no muscular involvement (amyopathic). Fevers and arthralgia or arthritis also are common features at presentation. Often, as in this patient, the extrapulmonary features of this disease are mild. The typical dermatomyositis skin manifestations of Gottron’s papules, mechanic’s hands, heliotrope rash, and shawl sign can occur. Features suggestive of a cutaneous vasculopathy—skin ulcers and palmar papules— also associated with anti-MDA5 dermatomyositis, and not with other dermatomyositis subtypes.
Anti-MDA5 dermatomyositis has a high mortality rate because of acute respiratory failure resulting from rapidly progressive interstitial lung disease. A retrospective French study looked at outcomes among patients with anti-MDA5 dermatomyositis and acute respiratory failure requiring ICU care between 2010 and 2017. Most of the patients had severe ARDS (Pao 2 to Fio 2 ratio of ≤ 100 mm Hg with positive end-expiratory pressure of ≥ 5 mm H2O) and all patients (n = 19) received corticosteroids and other immunosuppressants (84% cyclophosphamide, 32% rituximab, 11% tacrolimus, 11% cyclosporine, and 11% basiliximab). ICU mortality was 85%. Anti-Ro positivity, present in 60% of patients with anti-MDA5 dermatomyositis, and pneumomediastinum, reported in 15% of patients, both are associated with a poor prognosis.
Early treatment with aggressive combination immunosuppressant therapy is recommended. A multicenter study on combination immunosuppressant therapy in patients with anti-MDA5 dermatomyositis-associated interstitial lung disease showed a significantly higher 6-month survival compared with a historic group of patients who received step-up treatment (89% vs 33%). Combination treatment included high-dose corticosteroids, IV cyclophosphamide, and tacrolimus vs the step-up treatment of initial corticosteroid treatment with a later addition of other immunosuppressive agents. Cyclophosphamide typically is added in cases of life-threatening or refractory disease. Other treatments described in the literature include IV immunoglobulin and rituximab. IV immunoglobulin has been shown to be an effective treatment in idiopathic inflammatory myopathies when used at high doses, and rituximab is an effective treatment in dermatomyositis for lung, muscle, and skin disease.
Clinical Course
The subtle cutaneous features in keeping with dermatomyositis and anti-Ro positivity prompted a rheumatology opinion and extended myositis panel request. This confirmed the presence of anti-MDA5 antibodies. These findings are characteristic for anti-MDA5 dermatomyositis, which may have a fatal course. He was given IV immunoglobulin 2 g/kg over 48 h, pulsed with IV methylprednisolone 750 mg for 3 days, and then given rituximab and cyclophosphamide therapy in conjunction with a corticosteroid wean.
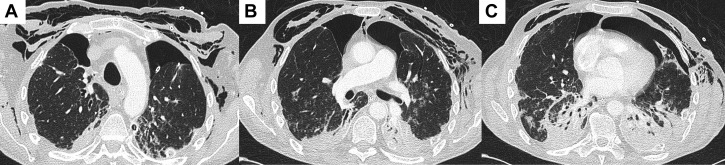
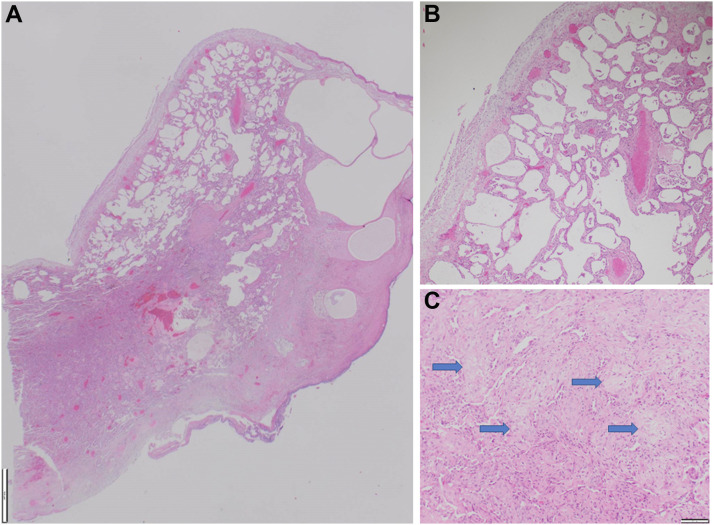
Despite treatment, he remained hypoxic and ventilator dependent. After resuscitation subsequent to a hypoxia-induced bradycardic arrest, chest radiography demonstrated a left-sided pneumothorax. A synchronous right-sided pneumothorax developed 1 week later with pneumomediastinum (Fig 4 ). He later underwent video-assisted thoracoscopic surgery pleurodesis for a persistent air leak on the left side. A lung biopsy sample was obtained at this time that revealed histopathologic findings consistent with nonspecific interstitial pneumonia and diffuse alveolar damage (Fig 5 ). Ventilatory wean was slow despite tracheostomy, and he experienced multiple episodes of ventilator-associated pneumonia. Unfortunately, after a 12-week stay in the ICU, this patient died after an episode of ventilator-associated pneumonia.
Figure 4.
A-C, Axial slices from a chest CT scan demonstrating progression of bilateral lower lobe consolidation and bilateral pneumothoraces with extensive subcutaneous emphysema throughout the superficial soft tissues of the chest extending into the mediastinum.
Figure 5.
A-C, Photomicrographs of video-assisted thoracoscopic surgery lung biopsy samples showing: (A) cystically dilated subpleural alveoli and rigid-appearing alveolar walls (×125 magnification; scale bar, 1,000 μm), (B) diffuse regular interstitial expansion of alveolar walls by fibrosis consistent with a nonspecific interstitial pneumonia pattern (×100 magnification; scale bar, 100 μm), and (C) focal organizing pneumonia with endoalveolar buds (blue arrows) also present (×100 magnification; scale bar, 100 μm).
Clinical Pearls
-
1.
Dermatomyositis subtypes are identified by skin and muscle findings, presence and progression of associated lung disease, and the presence of a myositis-specific antibodies (Table 1).
-
2.
Anti-MDA5 dermatomyositis is associated with rapidly progressive interstitial lung disease and cutaneous disease with little to no muscular involvement.
-
3.
Anti-MDA5 dermatomyositis has a high mortality rate because of acute respiratory failure resulting from rapidly progressive interstitial lung disease.
-
4.
Concurrent anti-Ro antibody positivity and the complication of pneumomediastinum both are associated individually with poor survival in anti-MDA5 dermatomyositis.
-
5.
Treatment should be initiated early with aggressive immunosuppression and should be combined with respiratory support.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Suggested Readings
- Sato S., Hirakata M., Kuwana M., et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52(5):1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- Fiorentino D., Chung L., Zwerner J., Rosen A., Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65(1):25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szodoray P., Hajas A., Kardos L., et al. Distinct phenotypes in mixed connective tissue disease: subgroups and survival. Lupus. 2012;21:1412–1422. doi: 10.1177/0961203312456751. [DOI] [PubMed] [Google Scholar]
- Sato S., Kuwana M., Fujita T., Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. 2013;23(3):496–502. doi: 10.1007/s10165-012-0663-4. [DOI] [PubMed] [Google Scholar]
- Ceribelli A., Fredi M., Taraborelli M., et al. Prevalence and clinical significance of anti-MDA5 antibodies in European patients with polymyositis/dermatomyositis. Clin Exp Rheumatol. 2014;32(6):891–897. [PubMed] [Google Scholar]
- Labrador-Horrillo M., Martinez M.A., Selva-O’Callaghan A., et al. Anti-MDA5 antibodies in a large Mediterranean population of adults with dermatomyositis. J Immunol Res. 2014;2014:290797. doi: 10.1155/2014/290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman D.J.B., Vleugels R.A. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78(4):776–785. doi: 10.1016/j.jaad.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Vuillard C., Pineton de Chambrun M., de Prost N., et al. Clinical features and outcome of patients with acute respiratory failure revealing anti-synthetase or anti-MDA-5 dermato-pulmonary syndrome: a French multicenter retrospective study. Ann Intensive Care. 2018;8(1):87. doi: 10.1186/s13613-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H., Nakashima R., Hosono Y., et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–498. doi: 10.1002/art.41105. [DOI] [PubMed] [Google Scholar]
- Zhou M., Ye Y., Yan N., Lian X., Bao C., Guo Q. Noninvasive positive pressure ventilator deteriorates the outcome of pneumomediastinum in anti-MDA5 antibody-positive clinically amyopathic dermatomyositis. Clin Rheumatol. 2020;39(6):1919–1927. doi: 10.1007/s10067-019-04918-2. [DOI] [PubMed] [Google Scholar]
- Mehta A.A., Paul T., Cb M., Haridas N. Anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease: report of two cases. BMJ Case Rep. 2021;14(4) doi: 10.1136/bcr-2020-240046. [DOI] [PMC free article] [PubMed] [Google Scholar]