FIG. 3.
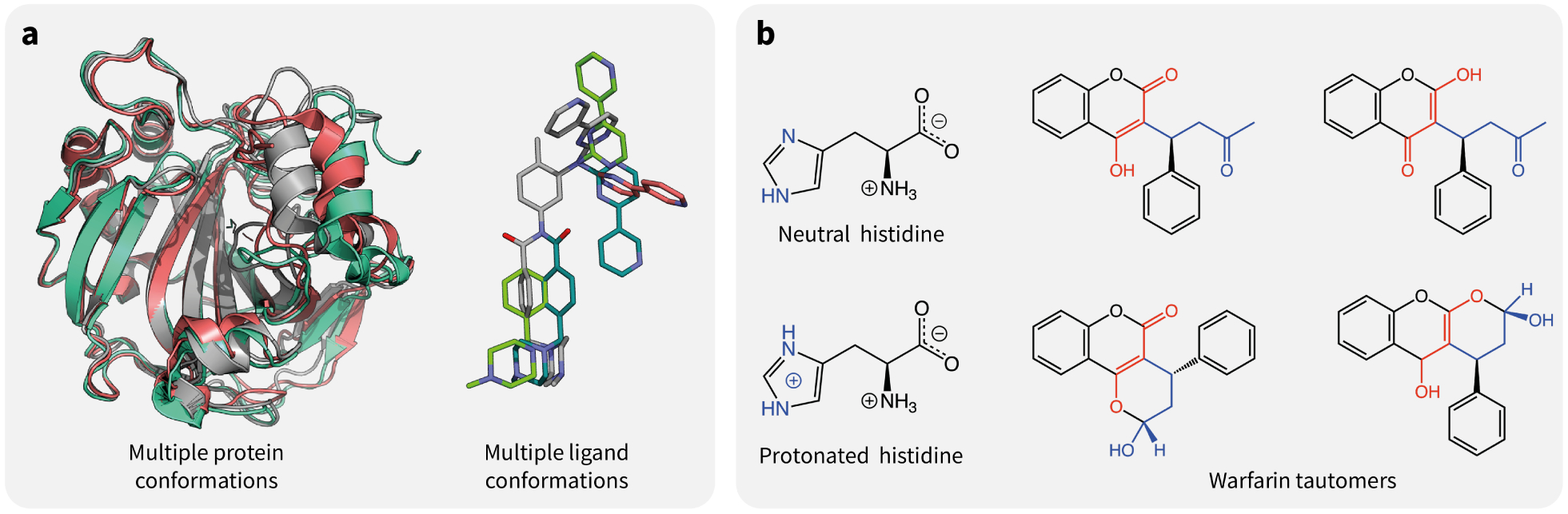
Examples of uncertainty in the structural and chemical inputs used by ML models. (a) Conformational variability is encountered both in proteins and small organic molecules. On the left-hand side, some of the conformations of carbonic anhydrase II are shown, as observed via nuclear magnetic resonance (PDB-ID 6HD2). On the right-hand side are some of the possible conformations of Imatinib (computed with Frog [56]). (b) Examples of major protonation and tautomeric states present in the solution for an amino acid and a drug. The protonated form of histidine has a pKa of approximately 6.0, which means that it is generally expected to be found in its neutral form at physiological pH. Yet, because of the local environment in protein binding pockets, it is often unclear which of the two is the major species, and they may both co-exist in non-negligible proportions. On the right-hand side, some of the tautomeric states of warfarin [57] are shown.
