Highlights
-
•
Proton therapy is a good treatment option for partial breast irradiation.
-
•
Proton PBI results in excellent local tumor control and OAR sparing.
-
•
Cosmesis and quality of life with PBT are comparable to other PBI modalities.
Keywords: Breast cancer, Proton therapy, Partial breast irradiation, Breast conservation therapy, Outcomes
Abstract
Purpose
Proton therapy (PT) for partial breast irradiation (PBI) in early-stage breast cancer can decrease morbidity versus photon PBI with superior organs-at-risk sparing. We report 3-year outcomes of the first prospective, multicenter, phase II trial of proton PBI.
Methods and Materials
This Proton Collaborative Group phase II trial (PCG BRE007-12) recruited women ≥ 50 years with node-negative, estrogen receptor (ER)-positive, ≤3cm, invasive ductal carcinoma (IDC) or ductal carcinoma in situ undergoing breast conserving surgery followed by proton PBI (40 Gy(RBE), 10 daily fractions). Primary endpoint was freedom from ipsilateral breast cancer recurrence. Adverse events were prospectively graded using CTCAEv4.0. Breast Cancer Treatment Outcome Scale (BCTOS) assessed patient-reported quality of life (PRQOL).
Results
Thirty-eight evaluable patients enrolled between 2/2013–11/2016. Median age was 67 years (range 50–79); 55 % had left-sided disease, and median tumor size was 0.9 cm. Treatment was delivered in ≥ 2 fields predominantly with uniform scanning PT (n = 37). At 35-month median follow-up (12–62), all patients were alive, and none had local, regional or distant disease progression. One patient developed an ER-negative contralateral IDC. Seven grade 2 adverse events occurred; no radiotherapy-related grade ≥ 3 toxicities occurred. Changes in BCTOS subdomain mean scores were maximum 0.36, indicating no meaningful change in PRQOL. Median heart volume receiving 5 Gy (V5Gy), lung V20Gy, and lung V10Gy were 0 %, 0 % and 0.19 %, respectively.
Conclusion
At 3 years, proton PBI provided 100 % cancer control for early-stage, ER-positive breast cancer. Toxicities are minimal, and PRQOL remains acceptable with continued follow-up. These findings support PT as a safe and effective PBI delivery option.
1. Introduction
Breast cancer diagnoses are more frequently being made at early stages of presentation. [1], [2] Breast cancer prognosis is excellent in early-stage breast cancer (EBC), leading to heightened awareness of long-term side effects and quality of life (QOL) preservation. [3], [4].
Breast conservation therapy (BCT) is a standard EBC treatment approach, allowing preservation of the affected breast and avoidance of more extensive surgery. Historically, breast conserving surgery (BCS) was followed by adjuvant whole breast irradiation (WBI) lasting 5–6 weeks, [5] but WBI is increasingly being delivering over shorter 3–4-week courses. [6], [7].
Partial breast irradiation (PBI) is an alternative approach for select patients with favorable EBC, allowing for further treatment acceleration. PBI is delivered with intracavitary balloon-based brachytherapy, interstitial needle-based catheter brachytherapy, intraoperative radiotherapy (IORT), and photon-based external beam radiotherapy, varying in duration from 1 fraction to 2 weeks. While these PBI techniques generally have favorable outcomes, there is inconsistency in cosmesis and toxicity. Since PBI is often employed to minimize toxicity, proton beam therapy (PBT) is a good alternative to further reduce long-term morbidity. [8], [9], [10], [11], [12], [13], [14], [15].
Proton PBI can improve heart, lungs and non-target breast tissue sparing compared with photon PBI due to the unique stopping power of the particle, as demonstrated in multiple comparative planning studies. [16], [17], [18] In one such study from investigators at MGH, nontarget breast tissue receiving 50 % of the prescribed dose was reduced by an average of 36 % with proton PBI compared with 3D-conformal photon PBI, and significant reduction in dose to the ipsilateral and contralateral lung and the heart were also seen. [16] Cardiac sparing with PBT is particularly noteworthy, as increasing evidence suggests long-term cardiac morbidity associated with incremental increases in heart radiation doses. [19], [20], [21] This may have particularly importance in breast cancer, where major coronary vessels traversing the heart surface often receive incidental irradiation and are susceptible to radiation damage that may result in future cardiovascular events. [22], [23].
Superior sparing of uninvolved ipsilateral and contralateral breast tissue, heart, and lung may result in a reduction in acute and late toxicities including soft tissue fibrosis, lung fibrosis, late cardiac sequelae, and radiation-related secondary malignancies. In EBC, with a cancer-specific survival rate > 95–99 %, QOL preservation and long-term morbidity reduction are critical [3], [4], [24], [25], [26].
The incidence of acute and late adverse effects of proton PBI remains undefined. Several institutional experiences have been reported promising but mixed results, with heterogeneity in treatment techniques and radiation doses used. [27], [28], [29], [30], [31], [32], [33] This study is the first EBC multicenter, prospective investigation of proton PBI.
2. Methods and Materials
2.1. Study design
BRE 007–12 is a phase II, multicenter, single arm Proton Collaborative Group (PCG) trial (clinicaltrials.gov identifier: NCT01766297). The PCG obtained IRB approval through Western IRB; institutional IRB approval for each enrolling institution was also obtained.
2.2. Patients
Eligible patients included females ≥ 50 years with newly diagnosed biopsy-proven, estrogen receptor (ER)-positive, Tis, T1, or T2 (AJCC, 7th and/or 8th Edition), node-negative invasive ductal carcinoma (IDC) or ductal carcinoma in situ (DCIS) ≤ 3 cm receiving BCT. Negative margin BCS was required (≥2mm from any invasive or in situ disease). Nodal staging (sentinel lymph node biopsy or axillary lymph node dissection) was performed for invasive cases. Exclusion criteria included: lobular histology, lymphovascular space invasion, BRCA 1/2 mutation, prior ipsilateral breast or thorax radiotherapy, and < 5-year life expectancy.
2.3. Procedures
PBI began within 12 weeks of BCS. Adjuvant endocrine therapy was administered at the treating medical oncologist’s discretion.
Fiducials or markers in the surgical bed were recommended and required if daily cone beam CT (CBCT) was unavailable. Daily orthogonal imaging was required at minimum for setup, with daily CBCT strongly encouraged. [34] CT simulation could be performed in the supine or prone position. When available, diagnostic breast MRI images were fused to simulation CT images for target delineation. Gross target volume (GTV) was defined as the surgical bed, including residual seroma. Clinical target volume (CTV) was a 1.5 cm radial expansion around GTV limited to pectoralis muscle and a skin-3 mm structure (avoidance volume defined as a 3 mm radial contraction from the skin). As this trial started before pencil beam scanning (PBS-PBT) widespread use, a traditional planning target volume (PTV) was added to account for uncertainty in treatment setup and patient movement (5 mm radial expansion around CTV, excluding pectoralis muscle and skin-3 mm structure).
Active scanning and passive scatter PBT were allowed. The prescription (Rx) dose was 40 Gy(RBE) delivered over two weeks in 10 daily fractions of 4 Gy(RBE) with ≥ 3 fields required for passive scattering plans. At least 2 fields were treated daily. Fewer fields could be utilized for active scanning plans if maximum skin dose was ≤ 100 % Rx dose. Excessively high MU spots were minimized from the skin contour. At least 95 % of the PTV was required to receive 100 % Rx dose, and 10 % of the PTV could receive up to 105 % Rx dose. Volume of the heart receiving 5 Gy (V5Gy) was constrained to < 5 %, while the ipsilateral lung V20 was < 1 % and V10Gy was < 5 %. The ipsilateral breast was constrained to V20Gy < 40 % and V40Gy < 35 %, while the contralateral breast dose constraint was V40Gy < 3 %.
Toxicities were scored CTCAEv4.0 and were assessed prior to treatment initiation, weekly during radiotherapy, 4 weeks after treatment completion, and annually thereafter. Patients completed the 22-point Breast Cancer Treatment Outcome Scale (BCTOS) questionnaire for QOL assessment prior to treatment initiation and at 1 and 3 years post-treatment [35] on a four-point Likert scale evaluating differences between the treated and untreated breast (1 = no difference, 4 = large difference). [36] Clinicians assessed cosmesis and photographs were obtained at baseline postoperatively prior to treatment and at 1 and 3 years (excellent, good, fair, poor). Tumor recurrence was assessed by clinical exam and annual mammography.
2.4. Outcomes
The primary study endpoint was freedom from failure (FFF), with failure defined as first ipsilateral breast cancer recurrence. The expected ipsilateral breast cancer recurrence rate was ≤ 3 %;the null hypothesis FFF ipsilateral breast cancer recurrence FFF will be ≤ 85 %, and the alternative hypothesis is ipsilateral breast cancer recurrence FFF ≥ 97 %. Sample size was calculated to achieve 95 % significance and power 80 %. Secondary endpoints included regional recurrence (occurring in the ipsilateral axilla, infraclavicular space, supraclavicular area, or internal mammary chain); distant recurrence; contralateral breast cancer occurrence; secondary primary cancer occurrence (non-breast); adverse events; cosmesis; and QOL.
2.5. Statistical Methods
Time-to event endpoints were estimated utilizing Kaplan-Meier methodology. Treatment-related toxicities were defined as increases in CTCAEv4.0 grade from pre-radiotherapy grade. BCTOS arithmetic means were calculated for all subdomains, with a change of 1 point or increase to an average of ≥ 3.0 (moderate change) constituted a clinically meaningful worsening in patient-reported QOL (PRQOL). Baseline adjusted scores were utilized to determine worsening cosmesis from baseline (change from excellent/good to fair/poor). Logistic regression was utilized to determine magnitude of correlation of socio-demographic, clinical, and treatment characteristics with worsening in cosmesis. Receiver operating characteristic (ROC) curve analysis and Youden’s were used to determine outcomes- and maximum likelihood-based optimum cutoff for DVH data.
3. Results
Forty-two women with ER positive, stage 0-II breast cancer were enrolled from 2/2013–11/2016. Three patients withdrew prior to treatment initiation, and 1 withdrew mid-treatment, resulting in 38 evaluable patients in this analysis. Median follow-up was 35 months (range, 12–62). Median patient age was 67 years (50–79) (Table 1). Twenty-one (55.3 %) patients had left-sided disease. Median tumor size was 0.9 cm (0.1–3.0). Most had an invasive component of disease (79.0 %) and grade 1 or 2 disease (86.8 %). Approximately-three-quarters of patients received HT (76.3 %); none received chemotherapy.
Table 1.
Patient and Tumor Characteristics (N = 38).
| Median | Range | |
|---|---|---|
| Length of Follow-up (months) | 34.5 | 11.5–70.1 |
| Age (years) | 67.0 | 50.0–79.0 |
| Laterality (n,%) | ||
| Right | 17 (44.7) | |
| Left | 21 (55.3) | |
| Biopsy Method (n,%) | ||
| Sentinel Lymph Node Biopsy | 29 (76.3) | |
| Axillary Dissection | 9 (23.7) | |
| T Stage (n,%) | ||
| Tis | 7 (18.4) | |
| T1a | 4 (10.5) | |
| T1b | 11 (28.9) | |
| T1c | 15 (39.5) | |
| T2 | 1 (2.6) | |
| Tumor Size (cm) | 0.9 | 0.0–3.0 |
| Histology (n,%) | ||
| IDC only | 15 (39.5) | |
| DCIS only | 8 (21.1) | |
| Mixed IDC + DCIS | 15 (39.5) | |
| Histologic Grade (n,%) | ||
| 1 | 14 (36.8) | |
| 2 | 19 (50.0) | |
| 3 | 5 (13.2) | |
| ER (n,%) | ||
| Positive | 38 (100.0) | |
| Negative | 0 (0.0) | |
| HER2/neu (n,%) | ||
| Positive | 4 (10.5) | |
| Negative | 34 (89.5) | |
| Hormonal Therapy (n,%) | ||
| Aromatase Inhibitor | 26 (68.4) | |
| Tamoxifen | 3 (7.9) | |
| None | 9 (23.7) | |
| BMI (kg/m2) | 29.3 | 19.6–45.3 |
| Bra Cup Size (n,%) | ||
| A | 2 (5.3) | |
| B | 9 (23.7) | |
| C | 8 (21.1) | |
| D | 9 (23.7) | |
| DD/E | 7 (18.4) | |
| DDD/F | 1 (2.6) | |
| H | 1 (2.6) | |
| Not Reported | 1 (2.6) |
Note: IDC = invasive ductal carcinoma; DCIS = ductal carcinoma in situ; ER = estrogen receptor; HER2/neu = human epidermal growth factor receptor 2; BMI = body mass index.
All patients completed the full 10-fraction treatment course as prescribed. There were no treatment interruptions due to toxicity. Uniform/active scanning PBT (US-PBT) was delivered for all excepting one patient who received passive scattering PBT (PS-PBT).
In total, 7 grade 2 events occurred as radiation dermatitis (RD) (n = 1, 2.6 %), lymphedema (n = 1, 2.6 %), hot flashes (n = 3, 7.9 %), dyspnea (n = 1, 2.6 %), and fatigue (n = 1, 2.6 %) (Table 2). Of these, RD and fatigue were probably or definitely related to radiotherapy. One grade 3 event was reported, cardiovascular disease requiring cardiac stent placement, the timing and nature of which informed an attribution of unrelated to radiation, particularly given that this patient had right-sided disease, heart V5Gy was 0 %, and it occurred only 29 days following PBI completion. Twenty-seven patients developed grade 1 RD (71.1 %); other radiation-related grade 1 toxicities included breast pain (n = 5, 13.2 %), fatigue (n = 8, 21.1 %), skin pain (n = 4, 10.5 %), lymphedema (n = 2, 5.3 %), telangiectasia (n = 1, 2.6 %), chest wall pain (n = 1, 2.6 %), and pruritis (n = 1, 2.6 %).
Table 2.
Adverse Events (N = 38).
| Adverse Event (Likely/Possibly Radiation-Related) | Number of Events (n, %) | ||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Skin Pain | 4 (10.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Radiation Dermatitis | 27 (71.1) | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| Telangiectasia | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 8 (21.1) | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| Chest Wall Pain | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Breast Pain | 5 (13.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lymphedema | 2 (5.3) | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| Pruritis | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Adverse Events (Unlikely Radiation- Related) | Number of Events (n, %) | ||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Vascular Ischemia | 0 (0) | 0 | 1 (2.6) | 0 (0) | 0 (0) |
| Hot Flashes | 10 (26.3) | 3 (7.9) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnea | 0 (0) | 1 (2.6) | 0 (0) | 0 (0) | 0 (0) |
| Cough | 3 (7.9) | 0 | 0 (0) | 0 (0) | 0 (0) |
| Back Pain | 2 (5.3) | 0 | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (2.6) | 0 | 0 (0) | 0 (0) | 0 (0) |
| Arthralgia | 2 (5.3) | 0 | 0 (0) | 0 (0) | 0 (0) |
On BCTOS, patients experienced the most change in nipple appearance, breast shape, scar tissue formation, breast texture, and bra fit. Without considering baseline pre-radiotherapy scores, five patients (13 %) assigned a BCTOS score of 4 at 1- or 3-year follow-up for change in nipple appearance (n = 3), breast shape (n = 4), scar tissue formation (n = 2), breast texture (n = 1) and bra fit (n = 1). Given that the mean scores and 95 % CI for each subdomain are below 2.0 with a maximum change of 0.36 points (Table 3), there was no clinically meaningful change in PRQOL according to the BCTOS measure in the domains of cosmetic status, functional status, and breast pain.
Table 3.
Means and 95% Confidence Intervals for Breast Cancer Treatment Outcome Scale (BCTOS) Domains.
| BCTOS QOL Domain | Baseline (N = 38) | 1 Year (N = 28) | 3 Years (N = 16) |
|---|---|---|---|
| BCTOS Aesthetic | 1.46 (1.33–1.58) | 1.53 (1.38–1.68) | 1.82 (1.50–2.14) |
| BCTOS Function | 1.16 (1.06–1.27) | 1.08 (1.02–1.15) | 1.24 (1.03–1.46) |
| BCTOS Sensitivity | 1.48 (1.36–1.61) | 1.38 (1.26–1.51) | 1.42 (1.22–1.62) |
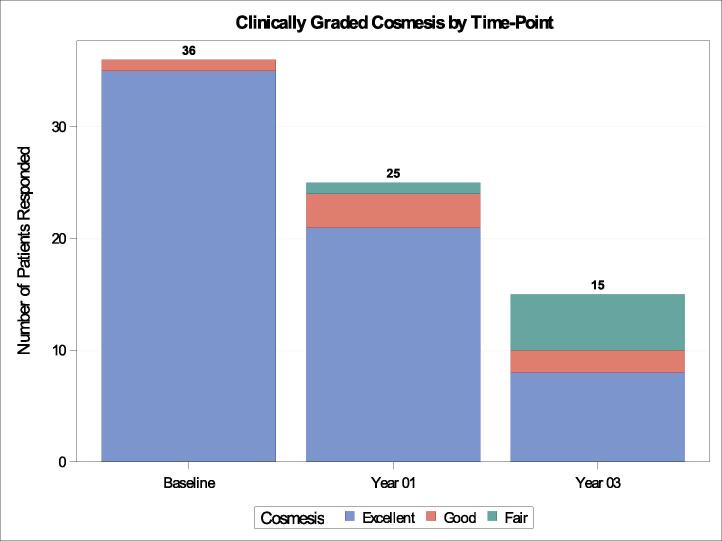
For clinician-reported assessment of overall breast cosmesis, there was expected attrition in evaluation completion from 36 patients (94.7 %) at baseline to 25 (65.8 %) at 1 year and 15 (39.5 %) at 3 years (Fig. 2). Prior to protocol treatment, all patients were given a score of “good’ (n = 1, 2.8 %) or “excellent” (n = 35, 97.2 %), which remained stable at 1 year (only 1 patient (4 %) was given a “fair” rating). At 3 years, of 15 assessments, a grade of “fair” was assigned to 5 patients (33.3 %), with the remainder still rated as “good” (n = 2, 13.3 %) or “excellent” (n = 8, 53.3 %). No patients received a score of “poor” over any timepoints.
Fig. 2.
Clinician-reported Cosmesis Over Time.
Breast V20Gy and V40Gy were significantly associated with decline in cosmesis scores over time (Table 4). On ROC analysis, Youden’s index demonstrated that volumes of breast receiving over 20 Gy of 30.5 % and volume of breast receiving over 40 Gy of 19.5 % remained significant for worsening cosmesis. Cosmesis was not associated with body-mass index (BMI), bra cup size, age, or tumor size.
Table 4.
Clinical and DVH Cut-off Parameters for Worsening Cosmesis.
| Variable | OR | p-value |
|---|---|---|
| Age | 1.11 (0.94 – 1.01) | 0.23 |
| BMI | 0.90 (0.74–1.10) | 0.31 |
| Axillary Dissection vs SLNBx | 1.79 (0.27 – 11.86) | 0.55 |
| Laterality - Right vs Left | 0.20 (0.02 – 1.91) | 0.16 |
| Histologic Grade (Ref = Grade 1) | ||
| Grade 2 | 2.44 (0.23 – 26.29) | 0.41 |
| Grade 3 | 8.66 (0.58–130.07) | 0.56 |
| Hormonal Therapy (Ref = None) | ||
| Tamoxifen | 3.00 (0.18 – 50.79) | 0.26 |
| AI | 1.00 (0.15 – 6.91) | 0.91 |
| Bra Cup Size ≥ D | 2.43 (0.38 – 15.27) | 0.34 |
| Tumor Size (cm) | 1.52 (0.46 – 5.00) | 0.49 |
| PTV D95% | 1.20 (0.43 – 3.39) | 0.73 |
| PTV Max Dose | 1.06 (0.67 – 1.68) | 0.80 |
| Lung V10Gy | 1.95 (0.72 – 5.25) | 0.19 |
| Breast V20Gy | 1.22 (1.03 – 1.44) | 0.02 |
| Breast V40Gy | 1.26 (1.03 – 1.54) | 0.03 |
| Breast V20Gy ≥ 30.48 % | 15.00 (1.52 – 148.31) | 0.02 |
| Breast V40Gy ≥ 19.48 % | 15.00 (1.52 – 148.31) | 0.02 |
Note: BMI = body-mass index; SLNBx = sentinel lymph node biopsy Ref = reference; AI = aromatase inhibitor; PTV = planning target volume; Gy = Gray.
Bold denotes statistical significance.
At last follow-up, there were no reported ipsilateral breast cancer recurrences or regional recurrences, and no deaths were reported, with local control, nodal disease-free survival and overall survival all 100 % at 3 years. One new primary cancer occurred in the contralateral breast, which was removed with wide local excision.
Heart V5Gy was 0 % (0.0–0.8), median lung V20Gy was 0 % (0.0–1.7) and V10Gy was 0.19 % (0.0–3.4), and V20 and V40 of the ipsilateral breast were 27.6 % (15.2–42.7) and 16.4 % (8.1–30.4), respectively.
4. Discussion
The potential clinical and dosimetric benefits of PBT have been clearly demonstrated in a number of disease sites, including craniospinal irradiation [37], [38], head and neck [39], [40], liver [41], [42], esophagus [43], and lung [44], [45], among others. For breast cancer, the advantage of PBT has primarily been shown for comprehensive nodal irradiation, with protons better able to spare heart and lung radiation exposure, while also delivering a more homogeneous target volume dose distribution. [16], [17], [18] The clinical impact of this dosimetric difference when delivery comprehensive nodal irradiation including the internal mammary chain is under active investigation in the RadComp/RTOG 3510 phase III study (NCT02603341).
Proton dosimetric advantages also exist for PBI. Heart, lung, nontarget ipsilateral and contralateral breast tissue are all spared excess irradiation compared with photons (Fig. 1). [46], [47], [48], [49] A dose-volume effect on breast cosmesis has been reported. In a prospective study from Liss and colleagues, PBI using IMRT to 38.5 Gy in 10 fractions was delivered twice daily (BID), after which a significant correlation in fair/poor cosmesis with greater breast volume receiving 20 %, 50 %, 80 %, and 100 % Rx dose was found. [50] A similar effect was demonstrated by Leonard et al. in 80 patients treated with the same dose using 3D conformal PBI, 19 % of whom had fair or poor cosmesis associated with breast volume receiving 50 %, 80 %, and 100 % Rx dose. [51] Borger and colleagues also noted a fourfold risk of fibrosis for every 100 cc increase in irradiated boost volume with iridium implant. [52] Thus, more conformal, tissue-sparing afforded with protons may be beneficial to improve cosmesis.
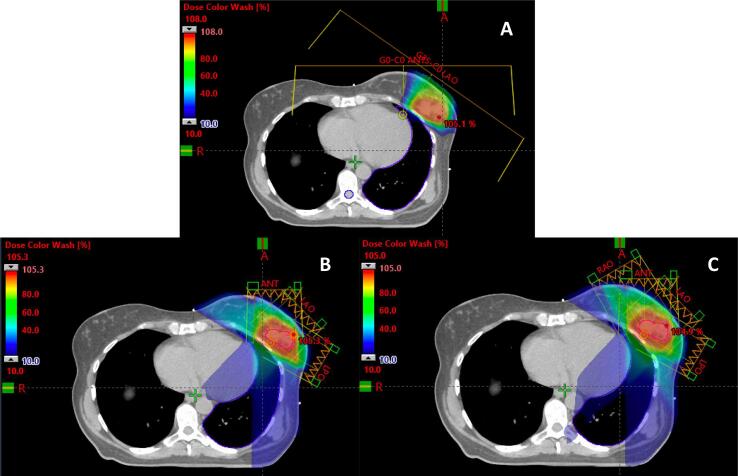
Fig. 1.
Proton and Photon Partial Breast Irradiation Treatment Plans. Representative patient with a left-sided breast cancer status post breast conserving surgery receiving partial breast irradiation. A) Representative axial slice of a pencil beam scanning proton therapy plan using two treatment fields (anterior and left anterior oblique). B) Representative axial slice of a photon static-field intensity-modulated radiation therapy (IMRT) plan using 3 fields (anterior, left anterior oblique, and left posterior oblique). C) Representative axial slice of a photon static-field intensity-modulated radiation therapy (IMRT) plan using 4 fields (right anterior oblique, anterior, left anterior oblique, and left posterior oblique). Lumpectomy cavity is outlined in red. Colorwash minimize dose of 10% prescription dose (blue) to maximum dose (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Cardiac sparing with PBT PBI is also potentially advantageous, particularly for left-sided and medial tumors. This is noteworthy given mounting evidence suggesting a direct relationship between late major coronary events and every incremental Gy of excess mean dose delivered to the heart. [20], [21].
Finally, PBT PBI may reduce secondary malignancy risk for both second breast and non-breast thoracic cancers. [24], [25], [53] In a systematic review and meta-analysis of 762,468 patients, the risk of second non-breast cancer, was higher in patients who received breast cancer radiotherapy. [26] Breast irradiation can also increase risk of contralateral breast cancer, although this risk may be tempered by the often more advanced age of patients receiving PBI. [25].
The feasibility of proton PBI and translating its dosimetric superiority to a clinically meaningful outcome improvement is an area of active investigation, including the PCG BRE007 trial. Three-year study results are very promising, particularly for disease control, with our results demonstrating 100 % local and regional disease control. QOL did not appear to meaningfully change in the three domains of cosmetic status, functional status, or breast pain. Clinician-reported cosmesis did have gradual decrease in scores of “good” and “excellent” at late timepoints; despite this, outcomes were comparable with those seen in the multicenter, randomized RAPID trial, in which fair/poor cosmesis was noted in 29 % of patients at 3 years and 36 % at 7 years. [15], [54].
Our results compare favorably with previous single-institutional studies. In an early phase I trial at Massachusetts General Hospital, 19 patients with stage I breast cancer were treated with PS-PBT using 1–3 fields to 32 Gy/8 BID fractions, with only 1 field treated per fraction, and an additional 79 patients treated with photons or mixed photons and electrons to the same dose. [27] Long term 7-year follow-up found the rate of physician-reported good-to-excellent cosmesis markedly worse with PBT (62 % proton versus 94 % photon/mixed, p = 0.03). [28] Acute and late skin toxicities was also higher with PBT.
In a phase II study from the National Cancer Center in South Korea, 30 patients were treated with PBT to 30 Gy(RBE)/5 daily fractions using 1–2 fields. [29] At 3 years, 69 % of patients achieved physician-reported good-to-excellent cosmesis. Increased toxicity was seen in those treated with a single field; 100 % of patients treated with 2 fields had good-to-excellent cosmesis. No local failures occurred.
Loma Linda University investigators treated 50 patients with PS-PBT on their phase II trial of small, early-stage IDC after BCS to 40 Gy(RBE)/10 daily fractions in 2–4 treatment fields. [30], [31] Only four patients experienced grade 2 acute toxicities. At 5 years, 90 % of patients had good-to-excellent cosmesis, and no local failures were noted.
Improved cosmesis and toxicities in the Loma Linda trial and our present trial are likely due to multiple factors. In contrast to these trials, in the NCC trial from Chang and colleagues, there was an increase in poor cosmesis when a single field was used vs two fields. [29] The use of only one field may have also contributed to the poorer cosmetic results in the MGH study. [27] In our and the Loma Linda trials, multiple beams were required, and our study required ≥ 2 fields treated per fraction, which may have resulted in superior cosmesis and toxicity. Similarly, in an interim analysis of a phase II trial from MD Anderson Cancer Center delivering 34 Gy(RBE)/10 BID fractions using PS-PBT, ≥2 fields were required, with 80 % of patients treated with a 3- or 4-beam arrangement, and at 2 years median follow-up, clinician- and patient-reported cosmesis was good or excellent in 87 % and 94 % of patients, respectively. [32] An early reporting of a prospective study of women receiving 3-fraction PBI with PBS-PBT to 21.9 Gy(RBE) and median two multi-field optimized beams from Mutter and colleagues noted good-to-excellent patient-reported outcomes in 98 % of patients and limited toxicities at 12 months. [33].
Radiation dose and timing also varied across studies. In the MGH and NCC experiences, daily dose delivered ranged from 6 to 8 Gy (4 Gy(RBE) BID). [27], [28], [29] Large fraction size and inadequate recovery time between fractions can increase risk of late effects [55], which, in combination with the number of fields treated per fraction, may worsen cosmesis. However, in the Mutter et al study, even with 9.3 Gy(RBE) fractions, favorable early outcomes was reported, possibly due to ample recovery between daily fractions and use of two-field plans. [33] In contrast, in the Loma Linda and this study, 40 Gy/10 daily fractions was used, and in the MD Anderson trial, 34 Gy(RBE)/10 BID fractions were used, respectively, both yielding excellent cosmesis and local control. [31], [32].
Finally, optimal PBT PBI dose and fractionation should be investigated. In our study, 40 Gy(RBE)/10 daily fractions (BED10 56.0; EQD2Gy 46.7) was utilized, akin to prior photon and proton PBI experiences. [31], [56] However, lower overall BED dose-fractionation regimens may achieve non-inferior local control, as was demonstrated in a University of Florence PBI trial (30 Gy/5 fractions, BED10 48.0, EQD2Gy 40.0); FAST-Forward trial (26 Gy/5 fractions to whole breast, optional tumor bed boost, BED10 39.5, EQD2Gy 32.9); and UK IMPORT LOW trial (40 Gy/15 fractions to partial breast, BED10 50.5, EQD2Gy 42.1). [12], [57], [58] Due to the higher linear energy transfer and differential RBE at the distal end of the Bragg peak with PBT and the 100 % control rates achieved in this and other proton studies, dose de-escalation warrants investigation. [59] Additionally, a once-daily treatment schedule may also be more favorable, allowing for improved long-term cosmesis relative to BID approaches. [55].
Cost effectiveness of proton PBI has been studied. [60], [61] Ovalle et al. performed a cost analysis of CPT codes for 8 frequently utilized treatment schedules and techniques for PBI and WBI. [62] Among 4 PBI approaches, multi-lumen brachytherapy had the highest total cost, while the cost of PBT and single lumen brachytherapy were within 11.4 % of each other, and 3D conformal photon therapy was the least costly. In the MD Anderson phase II interim analysis, median out-of-pocket cost for the PBI course was $700. [32] With limited treatment duration, trend towards decreased PBT cost, and low rates of toxicity and time away from work, proton PBI appears to be an increasingly cost-competitive treatment.
Patient selection is likely key for application of PBT PBI. PBT may be most optimal for patients with unfavorable anatomy who have tumor beds anterior to or near the heart where cardiac sparing is challenging using other radiation techniques, or for those where photons deliver excess dose in the contralateral breast and/or lung to achieve adequate target coverage. PBT may also be optimal for patients with large surgical beds or large CTV:nontarget ipsilateral breast tissue ratios, where the superior conformality of PBT can reduce the likelihood of adverse late cosmesis. Additional comparative and longitudinal studies of the impact of proton PBI versus other PBI modalities on dose to the surrounding normal structures including the cardiac substructures, coronary vessels, lung, contralateral breast, and non-target breast tissue will of value to better understand the absolute and relative difference of dosimetric sparing on these organs-at-risk. In the future, more ubiquitous application of model-based approaches for patient selection to identify those who will derive a substantial benefit from the enhanced normal tissue sparing possible with proton therapy will be of value to ensure optimal utilization of resources. [63] Further study to better understand ideal beam configurations will also be of value to further improve proton PBI delivery. While prior investigations have demonstrated that multi-field planning is critical [28], [29], it is unclear if specific beam angles or configurations should be considered to maximally spare breast tissue, minimize distal uncertainty, increase beam stopping in tissues unlikely to contribute to late toxicity, and allow for optimal conformality.
There are several limitations of this study. Post-treatment toxicity assessments were first performed at the 4-week timepoint. Prior studies, however, have reported acute side effects of hypofractionated radiotherapy may peak by 2 weeks post-treatment. [64] No patients were treated with more modern PBS-PBT, which has become standard in new proton centers. PBS-PBT can modulate skin surface dose and limit hotspots compared with PS-PBT and US-PBT, which may further minimize toxicities and better optimize cosmesis. [31], [49].
Although this is the first proton therapy multi-center prospective trial in breast cancer, the study sample was modest, thus limiting generalizability. Following full study accrual completion and data analysis, the study underwent an amendment to expand to 132 patients for statistical considerations taking into account interval published data of a lower recurrence rate than was originally cited for power calculation and estimated sample size required to fulfill the null hypothesis. The second phase of this trial is now reopened, and late outcomes of the original study population (n = 38) will continue.
5. Conclusion
Proton PBI is safe and effective, with excellent disease control, minimal toxicity, and acceptable patient- and physician-reported cosmesis and QOL outcomes on long-term follow-up. The improvement in heart, lung, and nontarget breast tissue achieved with proton PBI may decrease clinically significant late toxicities relative to photon PBT, including cardiovascular events, lung fibrosis, and secondary malignancies. This needs to be assessed in larger studies with long-term follow-up. With continued refinement and optimization of delivery and increased PBT patient access [65], this treatment approach has the potential to emerge as the modality that provides maximum benefit with the least toxicity for selected EBC patients.
Funding.
This research was supported by NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30-CA008748, period: 1/1/19 – 12/31/23.
Clinical Trials.gov Identifier.
Data Sharing Statement.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
J. Isabelle Choi, Email: ichoi@nyproton.com.
Todd DeWees, Email: DeWees.Todd@mayo.edu.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Ma J., Gaudet M.M., Newman L.A., Miller K.D., Goding Sauer A., et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . American Cancer Society; Atlanta, Ga: 2021. Cancer Facts & Figures 2021. [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(14)62240-6. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B., Anderson S., Bryant J., Margolese R.G., Deutsch M., Fisher E.R., et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 6.Whelan T.J., Pignol J.-P., Levine M.N., Julian J.A., MacKenzie R., Parpia S., et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 7.START Trialists' Group, Bentzen S.M., Agarwal R.K., et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuske R.R., Winter K., Arthur D.W., et al. Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: toxicity analysis of RTOG 95–16. Int J Radiat Oncol Biol Phys. 2006;65:45–51. doi: 10.1016/j.ijrobp.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U., Orecchia R., Maisonneuve P., et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomized controlled equivalence trial. Lancet Oncol. 2013;14(13):1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya J.S., Joseph D.H., Tobias J.S., et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomized, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 11.Strnad V., Ott O.J., Hildebrandt G., et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomized, phase 3, non-inferiority trial. Lancet. 2016;387(10015):229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 12.Livi L., Meattini I., Marrazzo L., Simontacchi G., Pallotta S., Saieva C., et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51(4):451–463. doi: 10.1016/j.ejca.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Vicini F.A., Cecchini R.S., White J.R., et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomized, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagsi R., Ben-David M.A., Moran J.M., et al. Unacceptable cosmesis in a protocol investigation intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelan T.J., Julian J.A., Berrang T.S., et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in site and node-negative breast cancer (RAPID): a randomized controlled trial. Lancet. 2019;394(10215):2165–2172. doi: 10.1016/S0140-6736(19)32515-2. [DOI] [PubMed] [Google Scholar]
- 16.Kozak K.R., Katz A., Adams J., Crowley E.M., Nyamwanda J.A., Feng J.K., et al. Dosimetric Comparison of Proton and Photon Three-Dimensional, Conformal, External Beam Accelerated Partial Breast Irradiation Techniques. Int J Radiat Oncol Biol Phys. 2006;65(5):1572–1578. doi: 10.1016/j.ijrobp.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Bush D.A., Slater J.D., Garberoglio C., Yuh G., Hocko J.M., Slater J.M. A technique of partial breast irradiation utilizing proton beam radiotherapy: Comparison with conformal X-ray therapy. Cancer J. 2007;13(2):114–118. doi: 10.1097/PPO.0b013e318046354b. [DOI] [PubMed] [Google Scholar]
- 18.Moon S.H., Shin K.H., Kim T.H., Yoon M., Park S., Lee D.-H., et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: Three-dimensional conformal radiotherapy, intensity modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol. 2009;90(1):66–73. doi: 10.1016/j.radonc.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Harris E.E.R., Correa C., Hwang W.-T., Liao J., Litt H.I., Ferrari V.A., et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast conservation treatment. J Clin Oncol. 2006;24(25):4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 20.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Brønnum D., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 21.Taylor C., Correa C., Duane F.K., Aznar M.C., Anderson S.J., Bergh J., et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correa C.R., Litt H.I., Hwang W.-T., Ferrari V.A., Solin L.J., Harris E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25(21):3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson G., Holmberg L., Garmo H., Duvernoy O., Sjögren I., Lagerqvist B.o., et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30(4):380–386. doi: 10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 24.Berrington de Gonzalez A., Curtis R.E., Gilbert E., Berg C.D., Smith S.A., Stovall M., et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102(1):220–226. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooning M.J., Aleman B.M.P., Hauptmann M., Baaijens M.H.A., Klijn J.G.M., Noyon R., et al. Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol. 2008;26(34):5561–5568. doi: 10.1200/JCO.2007.16.0192. [DOI] [PubMed] [Google Scholar]
- 26.Grantzau T., Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol. 2015;114(1):56–65. doi: 10.1016/j.radonc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Kozak K.R., Smith B.L., Adams J., Kornmehl E., Katz A., Gadd M., et al. Accelerated Partial Breast Irradiation Using Proton Beams: Initial Clinical Experience. Int J Radiat Oncol Biol Phys. 2006;66(3):691–698. doi: 10.1016/j.ijrobp.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Galland-Girodet S., Pashtan I., MacDonald S.M., Ancukiewicz M., Hirsch A.E., Kachnic L.A., et al. Long-term Cosmetic Outcomes and Toxicities of Proton Beam Therapy Compared With Photon-Based 3-Dimensional Conformal Accelerated Partial-Breast Irradiation: A Phase 1 Trial. Int J Radiat Oncol Biol Phys. 2014;90(3):493–500. doi: 10.1016/j.ijrobp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Chang J.H., Lee N.K., Kim J.Y., Kim Y.-J., Moon S.H., Kim T.H., et al. Phase II Trial of Proton Beam Accelerated Partial Breast Irradiation in Breast Cancer. Radiother Oncol. 2013;108(2):209–214. doi: 10.1016/j.radonc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Bush D.A., Slater J.D., Garberoglio C., Do S., Lum S., Slater J.M. Partial Breast Irradiation Delivered With Proton Beam: Results of a Phase II Trial. Clin Breast Cancer. 2011;11(4):241–245. doi: 10.1016/j.clbc.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Bush D.A., Do S., Lum S., Garberoglio C., Mirshahidi H., Patyal B., et al. Partial Breast Radiation Therapy With Proton Beam: 5-Year Results With Cosmetic Outcomes. Int J Radiat Oncol Biol Phys. 2014;90(3):501–505. doi: 10.1016/j.ijrobp.2014.05.1308. [DOI] [PubMed] [Google Scholar]
- 32.Pasalic D., Strom E.A., Allen P.K., Williamson T.D., Poenisch F., Amos R.A., et al. Proton Accelerated Partial Breast Irradiation: Clinical Outcomes at a Planned Interim Analysis of a Prospective Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2021;109(2):441–448. doi: 10.1016/j.ijrobp.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Mutter R.W., Jethwa K.R., Gonuguntla K., Remmes N.B., Whitaker T.J., Hieken T.J., et al. 3 fraction pencil-beam scanning proton accelerated partial breast irradiation: early provider and patient reported outcomes of a novel regimen. Radiat Oncol. 2019;14(1) doi: 10.1186/s13014-019-1417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiga C., Janssens G., Teng C.-L., Baudier T., Hotoiu L., McClelland J.R., et al. First Clinical Investigation of Cone Beam Computed Tomography and Deformable Registration for Adaptive Proton Therapy for Lung Cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):549–559. doi: 10.1016/j.ijrobp.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Stanton A.L., Krishnan L., Collins C.A. Form or function? Part 1. Subjective cosmetic and functional correlated of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2273–2281. [PubMed] [Google Scholar]
- 36.Feißt M., Hennigs A., Heil J., Moosbrugger H., Kelava A., Stolpner I., et al. refining scores based on patient reported outcomes – statistical and medical perspectives. BMC Med Res Methodology. 2019;19(1) doi: 10.1186/s12874-019-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St Clair W.H., Adams J.A., Bues M., Fullerton B.C., La Shell S., Kooy H.M., et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(3):727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 38.Brown A.P., Barney C.L., Grosshans D.R., McAleer M.F., de Groot J.F., Puduvalli V.K., et al. Proton Beam Craniospinal Irradiation Reduces Acute Toxicity for Adults With Medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86(2):277–284. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel S.H., Wang Z., Wong W.W., Murad M.H., Buckey C.R., Mohammed K., et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15(9):1027–1038. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 40.Blanchard P., Garden A.S., Gunn G.B., Rosenthal D.I., Morrison W.H., Hernandez M., et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer - A case matched analysis. Radiother Oncol. 2016;120(1):48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanford N.N., Pursley J., Noe B., Yeap B.Y., Goyal L., Clark J.W., et al. Protons versus Photons for Unresectable Hepatocellular Carcinoma: Liver Decompensation and Overall Survival. Int J Radiat Oncol Biol Phys. 2019;105(1):64–72. doi: 10.1016/j.ijrobp.2019.01.076. [DOI] [PubMed] [Google Scholar]
- 42.Hasan S., Abel S., Verma V., Webster P., Arscott W.T., Wegner R.E., et al. Proton beam therapy versus stereotactic body radiotherapy for hepatocellular carcinoma: practice patterns, outcomes, and the effect of biologically effective dose escalation. J Gastrointest Oncol. 2019;10(5):999–1009. doi: 10.21037/jgo.2019.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin S.H., Hobbs B.P., Verma V., et al. Randomized Phase IIB Trial of Proton Beam Therapy Versus Intensity-Modulated Radiation Therapy for Locally Advanced Esophgeal Cancer. J Clin Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins K.A., O'Connell K., Liu Y., Gillespie T.W., McDonald M.W., Pillai R.N., et al. National Cancer Database Analysis of Proton Versus Photon Radiation Therapy in Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2017;97(1):128–137. doi: 10.1016/j.ijrobp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Rwigema J.-C., Verma V., Lin L., Berman A.T., Levin W.P., Evans T.L., et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer. 2017;123(21):4244–4251. doi: 10.1002/cncr.30870. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald S.M., Jimenez R., Paetzold P., Adams J., Beatty J., DeLaney T.F., et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol. 2013;8(1) doi: 10.1186/1748-717X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez M.H., Zhang R., Sanders M., Newhauser W. A treatment planning comparison of volumetric modulated arc therapy and proton therapy for a sample of breast cancer patients treated with post-mastectomy radiotherapy. J Proton Ther. 2015;1(1):119. doi: 10.14319/jpt.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fagundes M.H.E.B., Pankuch M., et al. Proton therapy for local-regionally advanced breast cancer maximized cardiac sparing. Int J Particle Ther. 2015;1:827–844. [Google Scholar]
- 49.Wang X., Amos R.A., Zhang X., Taddei P.J., Woodward W.A., Hoffman K.E., et al. External-beam Accelerated Partial Breast Irradiation Using Multiple Proton Beam Configurations. Int J Radiat Oncol Biol Phys. 2011;80(5):1464–1472. doi: 10.1016/j.ijrobp.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liss A.L., Ben-David M.A., Jagsi R., Hayman J.A., Griffith K.A., Moran J.M., et al. Decline of Cosmetic Outcomes Following Accelerated Partial Breast Irradiation Using Intensity-Modulated Radiation Therapy: Results of a Single-Institution Prospective Clinical Trial. Int J Radiat Oncol Biol Phys. 2014;89(1):96–102. doi: 10.1016/j.ijrobp.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonard K.L., Hepel J.T., Hiatt R., et al. The Effect of Dose-Volume Parameters and Intrafraction Interval on Cosmetic Outcome and Toxicity After 3-Dimensional Conformal Accelerated Partial Breast Irradiation. Int J Radiat Oncol Biol Phys. 2013;85(3):623–629. doi: 10.1016/j.ijrobp.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 52.Borger J.H., Kemperman H., Sillevis Smitt H., Hart A., van Dongen J., Lebesque J., et al. Dose and volume effects on fibrosis after breast conservation therapy. Int J Radiat Oncol Biol Phys. 1994;30(5):1073–1081. doi: 10.1016/0360-3016(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 53.Vogel J., Lin L., Litzky L.A., Berman A.T., Simone C.B. Predicted Rate of Secondary Malignancies Following Adjuvant Proton Versus Photon Radiation Therapy for Thymoma. Int J Radiat Oncol Biol Phys. 2017;99(2):427–433. doi: 10.1016/j.ijrobp.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Mercieca-Bebber R., Friedlander M., Calvert M., et al. A systematic evaluation of compliance and reporting of patient-reported outcome endpoints in ovarian cancer randomized controlled trials: implications for generalizability and clinical practice. J Patient Rep Outcomes. 2017;1:5. doi: 10.1186/s41687-017-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bentzen S.M., Y J.R. Reports of unexpected late side-effects of accelerated partial breast irradiation – radiobiological consideration. Int J Radiat Oncol Biol Phys. 2010;77(4):969–973. doi: 10.1016/j.ijrobp.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braunstein L.Z., Thor M., Flynn J., et al. Daily Fractionation of External Beam Accelerated Partial Breast Irradiation to 40 Gy Is Well Tolerated and Locally Effective. Int J Radiat Oncol Biol Phys. 2019;104(4):859–866. doi: 10.1016/j.ijrobp.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunt A.M., Haviland J.S., Wheatley D.A., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomized, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coles C.E., Griffin C.L., Kirby A.M., et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomized, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaudhary P., Marshall T.I., Perozziello F.M., Manti L., Currell F.J., Hanton F., et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys. 2014;90(1):27–35. doi: 10.1016/j.ijrobp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Lundkvist J., Ekman M., Ericsson S.R., Isacsson U., Jönsson B., Glimelius B. Economic evaluation of proton radiation therapy in the treatment of breast cancer. Radiother Oncol. 2005;75(2):179–185. doi: 10.1016/j.radonc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Verma V., Shah C., Rwigema J.-C., Solberg T., Zhu X., Simone C.B. Cost-comparativeness of proton versus photon therapy. Chin Clin Oncol. 2016;5(4) doi: 10.21037/cco.2016.06.03. [DOI] [PubMed] [Google Scholar]
- 62.Ovalle V., Strom E.A., Godby J., Shaitelman S.F., Stauder M.C., Amos R.A., et al. Proton Partial-Breast Irradiation for Early-Stage Cancer: Is It Really So Costly? Int J Radiat Oncol*Biol*Phys. 2016;95(1):49–51. doi: 10.1016/j.ijrobp.2015.07.2285. [DOI] [PubMed] [Google Scholar]
- 63.Boersma L.J., Sattler M.G.A., Maduro J.H., et al. Model-Based Selection for Proton Therapy for Breast Cancer: Development of the National Indication Protocol for Proton Therapy and First Clinical Experiences. Clin Oncol. 2022;34:247–257. doi: 10.1016/j.clon.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Kole A.J., Kole L., Moran M.S. Acute radiation dermatitis in breast cancer patients: challenges and solutions. Breast Cancer Targets Ther. 2016;9:313–323. doi: 10.2147/BCTT.S109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maillie L., Lazarev S., Simone C.B., Sisk M. Geospatial Disparities in Access to Proton Therapy in the Continental United States. Cancer Invest. 2021;39(6-7):582–588. doi: 10.1080/07357907.2021.1944180. [DOI] [PubMed] [Google Scholar]