Abstract
Purpose
To establish a practical contouring strategy with reference atlases for the abdominopelvic bowel bag on treatment planning computed tomography (TPCT) and cone beam computed tomography (CBCT) images.
Methods and Materials
A scoping literature review was done to evaluate the existing definitions and contouring guidelines for bowel bag and small bowel planning-at-risk volume–like structures. A comprehensive definition was proposed for the abdominopelvic bowel bag that expanded the Radiation Therapy Oncology Group Pelvic Normal Tissue Consensus definition. Seven patients with TPCT and first-treatment-day CBCT images were selected from an institutional database to represent a range of normal anatomy and CBCT image quality. The TPCT and CBCT images were contoured using the proposed definition. During contouring, the Radiation Therapy Oncology Group definition's list of inclusion and exclusion structures was expanded. For areas with limited visibility of the bowel bag on either TPCT or CBCT, a set of operational definitions was developed based on consistently visible reference structures.
Results
A literature review showed that previously existing bowel bag definitions predominantly focused on the pelvic region and did not provide a complete and practical description of the full abdominopelvic contour relative to structures consistently visible in all radiation therapy images. The proposed contouring strategy had 4 components: a definition, a list of inclusion and exclusion structures, 15 tabulated operational definitions, and a set of atlases. The bowel bag was defined as the peritoneal cavity and retroperitoneal duodenum and ascending and descending colon, as visualized at the time of image acquisition. The operational definitions formalized the location of the peritoneal fascial planes through a simple look-up table. The proposed contouring strategy and reference atlases were successfully used on both TPCT and CBCT images.
Conclusions
This study produced a practical contouring strategy and reference atlases to enable reproducible delineation of the full bowel bag on TPCT and CBCT images. The strategy is a necessary first step toward consensus contouring with reduced observer variability, which is a prerequisite for evaluation of cumulative dose and its correlation with toxic effects, adaptive planning strategies, and automated contouring potential.
Introduction
The small and large bowel span the peritoneal and retroperitoneal abdominal and pelvic regions. They are large, mobile1 structures and are dose-limiting organs for many radiation therapy (RT) regimens owing to the risks of acute and long-term toxic effects.2, 3, 4 There are 2 main approaches to contouring a bowel planning-at-risk volume on treatment planning computed tomography (TPCT) images: the bowel bag and the individual bowel loops. The bowel bag can be described, conceptually, as all abdominal and pelvic regions that potentially contain small and large bowel. Radiation Therapy Oncology Group (RTOG) consensus contouring guidelines exist for normal anatomy in the pelvis5 and upper abdomen.6 The bowel bag approach is most commonly applied in the pelvis, whereas abdominal strategies and guidelines favor the loops approach. This has resulted in a relative deficit in guidance for contouring the bowel bag in the upper abdomen.
The small bowel spans the pelvis and upper abdomen and has been shown as particularly mobile.1 Studies have observed daily gross positional shifts of small bowel loops of up to 4 cm.7,8 Margins of up to 3 cm on individual loops were recommended to account for movement seen in 90% of patients.7,8 Assessment of the location of the small bowel during treatment could improve the accuracy of estimating the cumulative bowel dose and its correlation with toxic effects. However, the quality of the cone beam computed tomography (CBCT) images that are used for image guidance at the time of treatment is usually insufficient for contouring the individual loops manually.9 The bowel bag has inherently lower specificity than the individual loops. However, it may be better suited to account for mobile anatomy. Tuomikoski et al9 indicated that on-treatment CBCT image quality might be sufficient for bowel bag contouring in the pelvis. These points collectively support the need for the development of complete bowel bag contouring strategies for the upper abdomen and the pelvis that are suitable for use on both TPCT and CBCT images.
This study first summarizes existing bowel bag definitions and contouring guidelines from the literature. A new, comprehensive definition is then proposed and applied to contour a sample set of images. The new contouring strategy, accompanied with reference atlases, aims to accomplish 3 goals: (1) extend the existing contouring instructions for the pelvic bowel bag into the upper abdomen, (2) enable bowel bag contouring on both TPCT and CBCT images, and (3) improve contouring reproducibly to enable future development of useful ground truth and consensus contours. These goals are a prerequisite for evaluation of cumulative dose and its correlation with toxic effects, adaptive planning strategies, and automated contouring potential.
Methods and Materials
Literature review
A scoping literature review (PRISMA-ScR10) was performed to identify unique small bowel and bowel structure definitions using a bowel bag approach. PubMed, Web of Science, and major radiation oncology journals were searched independently by 2 authors (EO and KD) for the date range from January 1, 2000, to January 1, 2022, to identify primary articles. The predefined generic and Medical Subject Heading search terms used were small bowel, bowel, bowel bag, intestinal cavity, intestine, peritoneal cavity + bowel, and peritoneal space + bowel. Database searches also included “AND (radiation therapy OR radiation therapy).” Some mention of the bowel structure in the abstract was used as the criterion for reviewing the full article. Only English language articles were reviewed. Unique definitions and guidelines were then summarized and compared.
Patient image selection
Our institutional database was queried for patients with thoraco-lumbar, lumbar, and lumbo-sacral spinal metastases, treated between January and July 2016 with available first-day CBCT images of the abdominal-pelvic region. The TPCT and CBCT images for these patients were manually reviewed, and 7 patients were selected, 6 male and 1 female (rationale below). The mean time between acquisition of TPCT and CBCT scans was 2 weeks. All selected patients had been scanned and treated in the supine position without oral contrast and using a 3-mm scan slice thickness. Four-dimensional CT scans were not used. The selected patients met the following criteria: (1) they represented a range of normal anatomy—eg, obese versus cachectic, and with different levels of gastrointestinal (GI) and genitourinary (GU) structure fullness; (2) their TPCT images covered the full abdominal-pelvic region; (3) their CBCT images represented the clinically observed range of abdominal-pelvic image quality; (4) their CBCT images used the largest clinically available field of view (FOV; Elekta XVI M20: 26.0 cm axially, 41.0 cm diameter); and (5) their CBCT images had sufficient lateral anatomic coverage such that the abdominal wall was not truncated.
Development of a new contouring strategy
A new bowel bag definition was proposed based on the literature review. The inclusion and exclusion structures from the RTOG Pelvic Normal Tissue Contouring guidelines5 were modified and expanded. Operational definitions were developed to maximize contouring reproducibility by clearly identifying the boundary location relative to specific and visible anatomy. Contouring on TPCT and CBCT images was performed on each transverse image slice, starting inferiorly. Contours were performed by a single board-certified radiation oncologist, the chair of our institution's GI RT site group, who has >10 years of experience practicing in all GI anatomic subsites. The TPCT and CBCT images were assessed for consistent visibility of inclusion, exclusion, bounding, and reference structures.
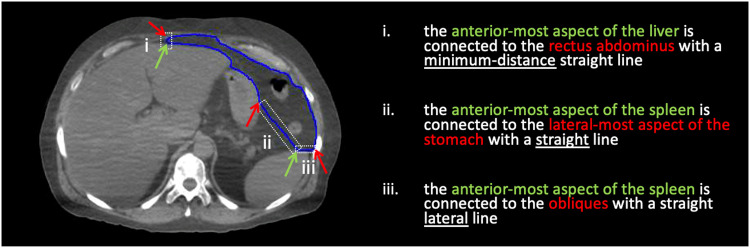
During the development of the contouring strategy, areas with limited visibility of known anatomic boundaries were identified in some locations in TPCT images and more broadly in CBCT images; an example for fascial planes is shown in Figure 1. To address this, a number of human sectional anatomy references (eg, Ellis et al11) were used to develop informed estimates of bowel bag boundary locations; then, operational definitions (ODs) were developed to reproducibly dictate the bowel bag boundary locations relative to reference structures in the areas with limited visibility. ODs indicate how to join easily identifiable points on reference structures that span the region of ambiguity with 1 of 3 line choices: straight, lateral, or minimum distance (Fig 2). The specific type of line connection between cardinal points on the reference structures was chosen to maximize reproducibility while maintaining a level of accuracy deemed clinically appropriate for all anatomies. The boundary locations dictated by the ODs are conservative, opting to include more volume rather than less.
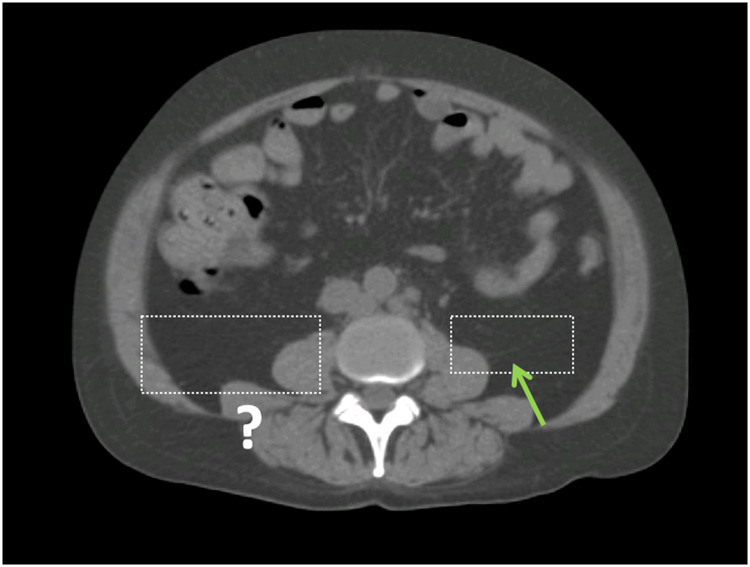
Fig. 1.
An example of limited visibility that led to the development of operational definitions in this study. The green arrow on the patient's left shows a faintly visible peritoneal fascial plane, which defines the bowel bag boundary. On the patient's right, the fascial plan is not visible, which requires the use of an operational definitions.
Fig. 2.
The 3 line types used to approximate the bowel bag boundary location.
Images from 3 of the 7 patients were used for the atlases as follows. The main atlas patient was a male whose CBCT was of good quality and with an FOV that fully covered the inferior boundary of the bowel bag. The planes and slices from this patient made up the main atlas. Given that the CBCT FOV is limited axially to 26.0 cm, a second male patient whose CBCT FOV was centered superiorly (including the diaphragm and beyond) was used to supplement the main atlas patient by providing TPCT and CBCT atlas coverage of the upper abdomen section. A third patient (a woman) was used to cover gender-specific pelvic anatomy (midfemoral heads to iliac crests). The 3 atlases included the contoured and the noncontoured images for both TPCT and CBCT for each selected slice. Transverse slices spanning the inferior- and superior-most contoured bowel bag were included in the atlases, along with 3 coronal and 5 sagittal planes. The full atlases are included as supplementary materials. Literature review results, contouring strategy development work, images, and atlases were all reviewed by gastrointestinal radiation oncologists from 3 outside institutions to ensure face validity of the work and to gather any feedback to incorporate on the strategy's recommendations.
Results
Summary of literature review
Thirty-five articles were found containing relevant structure definitions, 14 of which were unique.1,2,5,8,9,12, 13, 14, 15, 16, 17, 18, 19, 20 The earliest reference containing each unique definition is summarized in Table 1. The rest of this section presents our key observations from the literature review.
Table 1.
Summary of structures defined using a bowel bag approach*
| Reference | Disease site, No. of patients | Bowel naming | Definition |
|---|---|---|---|
| Muren et al, 200116 | Bladder, N = 25 | Small intestine | Volume potentially containing small intestinal tissue |
| Roeske et al, 200317 | GYNE, N = 50 | SB | Volume bounded by outermost extent of contrast-enhanced small bowel loops on all slices below the L4-5 interspace, explicitly excluding small bowel in upper abdomen |
| Cavey et al, 200518 | Prostate, N = 8 | IC | Conceptually described as contents of the intestinal cavity, bounded anteriorly and anterolaterally by the abdominal wall; posterolaterally by retroperitoneal and deep pelvic muscles; posteriorly by great vessels, vertebral bodies, sacrum, and rectum; and cranio-caudally, from top of iliac bones to most inferior slice with fat anterior to bladder; rectum excluded |
| Pollack et al, 200619 | Prostate, N = 100 | Bowel | Region of potential small bowel and distal colon and/or sigmoid |
| Price et al, 200620 | Prostate, N = 10 | Bowel | Conceptually described as including all space potentially occupied by bowel, ie, region between pelvic nodal areas from the sigmoid flexure, just above the rectum inferiorly, to 1 slice above most superior (periprostatic, periseminal vesicle, external iliac, proximal obturator, and proximal internal iliac, presacral/perirectal) lymph nodes |
| Gunnlaugsson et al, 200712 | Rectum, N = 28 18 M/10 F | Whole abdomen | Entire abdominal contents, explicitly including small bowel, large bowel, mesenteric structures, and abdominal fat and excluding liver, kidneys, spleen, large vessels, and psoas muscles |
| Sanguineti et al, 20081 | Prostate, N = 9 | IC | Conceptually described as the container, versus the content, acknowledging bowel loops are physically confined within the IC; the IC is bounded anteriorly by the abdominal/pelvic anterior wall, laterally by the pelvic wall, and inferiorly by the rectum and/or bladder |
| Fiorino et al, 20092 | Prostate, N = 175 | IC | IC by Sanguineti et al 20081 but excluding 5- to 7-mm margin around PTV; whole intestinal cavity (WIC) = IC Sanguineti et al 2008,1 N = 20 |
| Tuomikoski et al, 20119 | Bladder, N = 5 | IC | Conceptually described as abdominal cavity volume, limited anteriorly and laterally by the abdominal/pelvic wall and inferiorly by the rectum/bladder; explicitly including all visible bowel loops |
| Hysing et al, 20118 | Prostate, N = 3 | IC | Conceptually described as least specific PRV for small bowel, the physical boundary, the intestinal cavity; volume from the slice above L5, superiorly, to the slice where pubic bones meet, inferiorly; bounded anteriorly and laterally by abdominal and pelvic wall and posteriorly by deep muscles of back and pelvic bones |
| Gay et al, 20125 | GU/GYN, N = 2, 1 M/1 F | Bowel NOS BowelBag |
Bowel NOS (non-GI definition): peritoneal space occupied or potentially occupied by large or small bowel; BowelBag: abdominal contents, excluding muscle, bone, and all overlapping non-GI structures, inferiorly bounded by most inferior of the inferior-most small or large bowel loop or superior limit of rectum or ano-rectum; rectum and ano-rectum in the same slice as, or superior to, the inferior-most small or large bowel loop should be included; superiorly, the volume is extended 1 - 5 cm superior of PTV |
| Banerjee et al, 201313 | Rectum, N = 67, 38 M/29 F | PS | Conceptually described as the area where small or large bowel may lie at any point during treatment; volume bounded anteriorly and laterally by posterior aspect of abdominal muscles and posteriorly by vertebral bodies, sacrum, or posterior aspect of peritonealized sigmoid colon; inferiorly boundary 1 slice below inferior-most small bowel loop and superior boundary 5 slices superior to treatment plan field edge; all contoured small and large bowel explicitly included and bladder, prostate, ovaries, and uterus excluded |
| Pollack et al, 2015; RTOG 053414 | Prostate, N = 1764 | Potential bowel space | Conceptually described as the small and large bowel's potential space within the pelvis, including regions, laterally, on either side of bladder to medial edge of lymph node outline; bounded inferiorly by top of prostate bed and superiorly by superior-most slice of nodal CTV; presacral lymph node region explicitly excluded |
| Jhingran et al, 2012; RTOG 041815 | GYNE N = 92 | Small bowel | Conceptually described as the area where bowel may lie at any point during treatment; volume bounded by edge of the peritoneum, surrounding all small bowel loops and defined to a minimum of 2 cm superior of PTV |
Abbreviations: CTV = clinical target volume; GI = gastrointestinal; GU = genitourinary; GYNE = gynecologic; IC = intestinal cavity; NOS = not otherwise specified; PRV = planning-at-risk volume; PS = peritoneal space; RTOG = Radiation Therapy Oncology Group; SB = small bowel.
For RTOG trial protocols, the number of patients is the expected accrual.
The majority of the definitions in the literature were in the context of developing a planning-at-risk volume structure for pelvic target volume RT planning. Overall, bowel structures in the literature were described either conceptually or anatomically. The conceptual definitions used the space potentially occupied by the small and/or large bowel at any time during the treatment or at the time of imaging. The anatomic definitions used the content of the peritoneal, intestinal, and abdominal cavity or space. Roeske et al17 specifically indicated, and demonstrated in their Figure 3, a volume smaller than the peritoneal space. Gunnlaugsson et al12 described a volume larger than the peritoneal space by explicitly including abdominal fat. Some definitions explicitly listed inclusion and exclusion structures. Other definitions provided a boundary location relative to a specific aspect of an exclusion structure. The number of bounding structures listed was highly variable, and the structures provided typically depended on the treatment and/or disease site. Banerjee et al13 provided the most complete list in the pelvic region. Gunnlaugsson et al12 explicitly referenced abdominal organs that extend above the pelvis. The rest of the literature referenced only pelvic structures or used generalizations—eg, Gay et al5 excluded muscles, bones, and non-GI structures. Some definitions were accompanied by a figure—eg, Banerjee et al13 and Gay et al (RTOG pelvic guidelines)5 gave figures and atlases confined to the pelvic region below the iliac crests. The studies by Banerjee et al13 and Jhingran et al were the only 2 that included a temporal aspect in the definition by stating that their definitions applied to the potential bowel location at any time during treatment. Gay et al (RTOG pelvic guidelines)5 provided a thorough description of the inferior boundary location relative to the patient anatomy. All descriptions of the superior boundary were relative to the RT pelvic target volume or treatment field edge, not patient anatomy. The study by Tuomikoski et al9 was the only study that applied the authors’ definition to CBCT images, confined to the pelvic region. None of the definitions specifically addressed the location or shape of the boundary in areas with limited contrast.
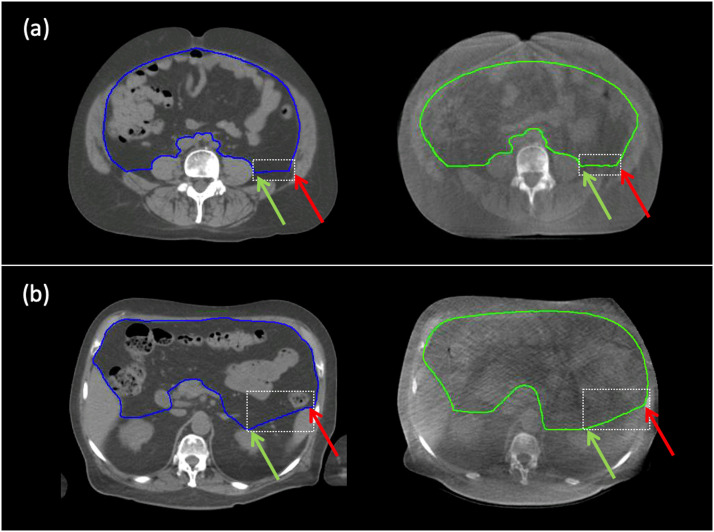
Fig. 3.
Examples of operational definitions operational definitions on treatment planning computed tomography (left) and cone beam computed tomography (right) within the dashed white boxes. A, operational definitions #1: if the peritoneal fascial plane is not visible in the lateral direction relative to the psoas major, then draw a lateral line from the lateral-most aspect of the psoas major to intersect the iliacus and contribute to the posterior boundary of the bowel bag. B, operational definitions #5: if the peritoneal fascial plane is not visible in the left lateral direction relative to the kidney, then draw a straight line from the anterior-most aspect of the kidney to the anterior-most aspect of the spleen to contribute to the posterior boundary of the bowel bag. Green and red arrows indicate, respectively, the relevant aspects of the origin and terminal structures.
The RTOG Normal Tissue Pelvis Consensus guidelines and atlases5 may be sufficient for reproducible contouring of the bowel bag in the pelvic region on TPCT images. However, they can benefit from additional guidance, particularly with the various definitions that exist in the literature. Additional guidance is needed for bowel bag contouring in the upper abdominal region on both TPCT and CBCT images and in the pelvic region on CBCT images.
Summary of the proposed contouring strategy
Our proposed strategy has 4 components: a definition, a list of inclusion and exclusion structures, 15 tabulated operational definitions, and a set of atlases. The following subsections summarize each of those components.
Bowel bag definition
We propose defining the bowel bag as the space potentially occupied by small and large bowel loops at the time of image acquisition. Our definition means that the bowel bag includes the peritoneal cavity plus the partially retroperitoneal duodenum and ascending and descending colon. In our definition, the RTOG Pelvis Consensus description of the inferior boundary is used (the most inferior small or large bowel loop or above the rectum or anorectum, whichever is most inferior). To remain consistent, the superior boundary is defined as the most superior small or large bowel loop, or below the diaphragm. The peritoneal fascial plane therefore represents the majority of the radial boundary. In the relevant axial range, the radial boundary is extended to include the duodenum and ascending or descending colon. The radial boundaries are modified to exclude nongastrointestinal structures within the peritoneal cavity (liver, gallbladder, and spleen), which is consistent with the RTOG Pelvis Consensus definition that excludes other pelvic organs.
Inclusion and exclusion structures
The following is the full list of inclusion and exclusion structures. Structures to be included are all small and large bowel loops and the peritoneal space, along with the mesorectal fat and rectum if they are colocated axially in slices with the inferior-most loops of bowel. Structures to be excluded are muscles (eg, rectus abdominus, transverse abdominus, obliques, psoas, obturator internus, and quadratus lumborum), bones, vessels (eg, external iliacs, internal iliacs, common iliacs, obturator vessels, aorta, inferior vena cava, superior mesenteric vessels, and renal vessels), bladder, prostate, seminal vesicles, rectum (if not colocated with inferior-most bowel loops), kidneys, liver, spleen, pancreas, stomach, adrenal glands, mesorectal fascia, inguinal ligament, retroperitoneal fascia, retroperitoneal fat, perirenal fascia, and perirenal fat.
ODs
Fifteen unique ODs are proposed in a simple look-up table (Table 2). The ODs are concentrated in the high pelvis and abdominal regions and mainly define the posterior and posterior-lateral boundaries. The table offers a standardized sentence format as follows: If the peritoneal fascial plane is not visible in the direction relative to the origin structure, then draw a line type from the X-most aspect of the origin structure to the Y-most aspect of the terminal structure to contribute to the bowel bag boundary of the bowel bag. Examples of the use of ODs are shown in Figure 3. The ODs should be ranked as lowest in priority relative to all other components of the strategy—examples are shown in Figure 4.
Table 2.
List of operational definitions (ODs)*
| OD# | Direction | Origin structure | X-most aspect of origin structure | Line type | Terminal structure | Y-most aspect of terminal structure | Bowel bag boundary | TPCT slice ref. # | CBCT slice ref. # |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lateral | Psoas major | Lateral-most | Lateral | Transverse abdominis or obliques or iliacus or quadratus lumborum | N/A | POST | MM-p26 PF-p113 PF-p115 | PF-p113 PF-p115 |
| 2 | Lateral | Kidney | Anterior-most | Lateral | Transverse abdominis or obliques or iliacus or quadratus lumborum | N/A | POST | MM-p36 UA-p68 | MM-p36 UA-p68 |
| 3 | Medial | Kidney | Anterior-most | Straight | Central vessels or aorta or IVC | Lateral-most | POST | MM-p35 UA-p69 | MM-p35 UA-p69 |
| 4 | Lateral (R) | Kidney | Anterior-most | Lateral | Liver | N/A | POST | MM-p40 UA-p73 | UA-p73 |
| 5 | Lateral (L) | Kidney | Anterior-most | Straight | Spleen | Anterior-most | POST | MM-p43 UA-p76 | UA-p76 |
| 6 | Medial (L) | Kidney | Anterior-most | Straight | Pancreas | Lateral-most | POST | MM-p47 UA-p75 | |
| 7 | Medial (L) | Kidney | Anterior-most | Straight | Stomach | Lateral-most | POST | MM-p48 | |
| 8 | Medial | Liver | Posterior medial-most | Straight | Central vessels or IVC | Lateral-most | POST | MM-p45 | UA-p77 |
| 9 | Lateral (R) | Liver | Anterior-most | Lateral | Transverse abdominis | N/A | POST/LAT | MM-p41 | |
| 10 | Anterior | Liver | Anterior-most | Minimum distance | Rectus abdominis | N/A | ANT/LAT | MM-p51 UA-p84 | UA-p84 |
| 11 | Lateral | Spleen | Anterior-most | Lateral | Transverse abdominis or obliques | N/A | POST | MM-p46 UA-p83 | UA-p83 |
| 12 | Medial | Spleen | Anterior-most | Straight | Pancreas | Lateral-most | POST | MM-p50 UA-p79 | UA-p79 |
| 13 | Medial | Spleen | Anterior-most | Straight | Stomach | Lateral-most | POST | MM-p52 | UA-p81 |
| 14 | Anterior | Stomach | Anterior-most | Straight | Liver | Left lateral-most | ANT | MM-p54 | |
| 15 | Lateral (L) | Stomach | Lateral-most | Straight | Pancreas | Left lateral-most | POST | MM-p49 UA-p80 | UA-p80 |
Abbreviations: ANT = anterior; CBCT = cone beam computed tomography; IVC = inferior vena cava; L = left; LAT = lateral; N/A, not applicable; OD = operational definition; POST = posterior; R = right; TPCT = treatment planning computed tomography.
The color coding is based on the origin structure. Atlas slice index references: MM = main male, UA = upper abdominal, PF = pelvic female, and P# = page number in the atlas.
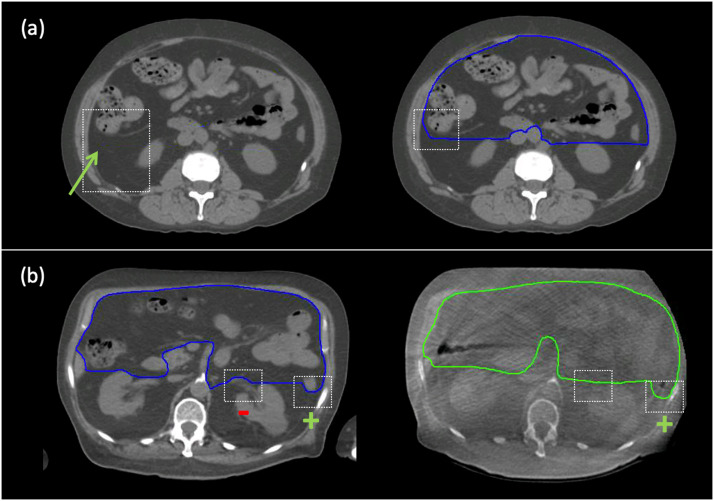
Fig. 4.
Two examples of the hierarchy in the application of the contouring strategy within the dashed white boxes. A, The left panel shows the partially visible fascial plane on treatment planning computed tomography that overrides operational definitions #2. The application of this hierarchy is shown in the right panel. B, The left panel shows that the inclusion of the bowel loop (denoted by a green +) outranks operational definitions #2, and the exclusion of the renal vessels (denoted by a red –) outranks OD#3. For the corresponding cone beam computed tomography slice in the right panel, there is insufficient cone beam computed tomography contrast for the exclusion of the renal vessels.
Atlases
A summary of atlas coverage is shown in the supplementary materials. The 3 atlases are provided as an electronic supplement. The atlases contain coronal and sagittal slices that show the axial extent of the bowel bag and show the position of each axial slice in the atlas relative to the gross anatomy. The axial slices are shown for the coregistered TPCT and CBCT images with and without contours to avoid the contours masking faint relevant anatomy features such as the fascial planes. Each OD is highlighted with text in the atlases.
Discussion
Practical use of the proposed contouring strategy
Our new definition of the bowel bag differs from previously published definitions in the following respects: (1) it avoids referencing the abdominal cavity, whose physical interpretation may vary; (2) it explicitly addresses sections of bowel that fall in the retroperitoneal space; (3) it describes all boundaries, including the inferior and superior boundaries, strictly relative to normal-tissue anatomic structures without referencing RT targets or dose distribution; and (4) it provides a specific point in time at which the definition should be applied, eliminating the need to infer the potential mobility of structures at boundaries.
Although the strategy covers the full bowel bag, it can be applied to any subsection. The wording of the bowel bag definition differs slightly from the RTOG Pelvic Normal Tissue Consensus atlases in 2 respects to improve clarity and consistency: (1) the proposed strategy uses the peritoneal cavity to describe the physical extent of potential small and large bowel loop position instead of the abdominal contents and (2) the proposed strategy explicitly states that it should be applied to the structure location at the time of image acquisition.
The following sequence should be used by an observer wishing to use the proposed contouring strategy: (1) Begin by deciding the axial range of interest. (2) Review the bowel bag definition to determine whether your axial range of interest spans the inferior-most and/or superior-most limits of the bowel bag, and, if required, apply the description of these locations from the definition to find the first and last slices of interest in your scan. (3) Review the list of inclusion and exclusion structures. (4) Begin contouring the peritoneal space inferiorly. (5) Whenever the peritoneal and/or renal fascial planes are not visible, determine the relevant origin structures from the ODs in Table 2 (the first row is inferior-most and the last row is superior-most). The last column in Table 2 refers to atlas slices that provide a visual example of the relevant ODs. (6) If implementing an operational definition from Table 2 causes exclusion of a visible inclusion structure or inclusion of a visible exclusion structure, then modify your contour to wrap around the visible extent of that structure, using atlas examples.
In the ODs, the straight-line approach demonstrates a clear preference for reproducibility over exact anatomic accuracy—an example is shown in Figure E6 a of the supplementary materials. In the abdominal region, straight lines seem to approximate the true anatomic location better because they are not straight lateral unless spanning only a small distance. The choice of the cardinal aspects of the reference structures is made based on the range of normal anatomy observed. For instance, as shown in Figure E6 b of the supplementary materials, the visible retroperitoneal planes in the upper pelvis were typically found farther posterior along the psoas muscle in cachectic patients versus extending off the anterior aspect in the normal-obese patients. For this reason, in OD#1, the lateral-most aspect of the psoas muscle was used as the average between these 2 extremes. Similarly, for kidney-based ODs (OD#2-7), the anterior-most aspect of the kidney was used as the straight-line connection point because the thickness of pararenal fat is variable.
CBCT structure visibility
The visibility of anatomic structures varies between and within CBCT images. Noise affects the visibility for larger patients (Fig E6 c of the supplementary materials). Cachectic patients have limited intra-abdominal and intraperitoneal fat and space, which limits the contrast between individual organs (Fig E6 d of the supplementary materials). Mobile gas causes artifacts that obstruct visibility locally (Fig E6 e of the supplementary materials). However, contour interpolation from adjacent slices can mitigate the effect of artifacts. Gas artifacts can be useful in differentiating (but not delineating) bowel loops (small gas bubbles) from the stomach (large gastric bubble). When visible, small gas artifacts are useful in detecting the superior-most or inferior-most bowel loops. The pancreas is referenced by ODs, but it is not consistently visible in CBCT images. However, minimal bowel bag volume changes were observed when OD#7 (stomach-kidney) was used instead of the stomach-pancreas-kidney combination (OD#15 and OD#6) (Fig E6 f of the supplementary materials). Overall, our review of clinical CBCT images supports the conclusion of Tuomikoski et al9 that the visualization of small bowel loops on CBCT images is variable and insufficiently consistent for contouring, whereas the majority of the bowel bag boundary can be consistently identified for contouring on CBCT images.
Limitations of the contouring strategy
There are some areas that present contouring challenges. In the low pelvis, the anterior-lateral pocket contiguous with the anterior abdominal wall (lateral termini of the rectus abdominus) can be delineated by estimating the location of the inguinal ligament; the ligament is often difficult to see even on diagnostic imaging. The locations of the junction between the mesorectum and sigmoid and the junction between the duodenum and stomach are difficult to identify despite detailed descriptions in the RTOG Pelvic and Upper Abdominal Normal Tissue guidelines, respectively. Exclusion of some pelvic organs on CBCT images can prove challenging, as can the exclusion of the mesenteric vessels in the pedicle region. Patients who have had viscera removed surgically will also have altered anatomy that can complicate the application of the strategy. Tests using our patient scans with mock hepatectomies and pancreatectomies have shown the ODs to be robust to alterations of this sort, but undoubtedly, as the strategy is used more, there will be circumstances that highlight the need to modify the ODs.
Future direction
The proposed contouring strategy forms the basis for our forthcoming study on inter- and intraobserver variability on contouring the bowel bag on TPCT and CBCT images, which has never been reported previously. Consensus contours from multiple expert observers with small observer variability is the basis for evaluating the cumulative dose to the bowel bag and its correlation with toxic effects, for evaluating adaptive planning strategies, and for assessment of automated contouring potential. Importantly, this strategy for contouring can work in conjunction with existing maximum point dose constraints commonly used in stereotactic ablative body-RT. Understanding the outcomes of low-dose splash areas for acute and long-term toxic effects for abdominopelvic stereotactic ablative body is an important relative knowledge gap in the growing field of curative- and palliative-intent stereotactic ablative body. The common use of 4-dimensional or slow CT for upper abdominal RT also makes delineating individual loops of bowel difficult and supports the use of a strategy anchored in reliably visible reference structures such as those defined in our strategy.
Conclusions
This study produced a practical contouring strategy and reference atlases for more reproducible contouring of the full bowel bag on TPCT and CBCT images. The main novel aspects are the inclusion of the upper abdomen in addition to the pelvis, consideration for CBCT images, and the use of ODs to approximate contours in areas of limited visibility. There are 4 components to the strategy: a definition, a list of inclusion and exclusion structures, ODs, and a set of atlases. The atlases are provided as a supplemental electronic resource.
Footnotes
Sources of support: This work was partially funded by NSERC Discovery Grant #2017-06253, held by Dr Ali.
Disclosures: none.
All data generated and analyzed during this study are housed within a secure institutional database. Interested readers can contact the study authors with questions regarding access or reuse.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.101031.
Appendix. Supplementary materials
References
- 1.Sanguineti G, Little M, Endres EJ, Sormani MP, Parker BC. Comparison of three strategies to delineate the bowel for whole pelvis IMRT of prostate cancer. Radiother Oncol. 2008;88:95–101. doi: 10.1016/j.radonc.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Fiorino C, Alongi F, Perna L, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:29–35. doi: 10.1016/j.ijrobp.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 3.Alongi F, Fiorino C, Cozzarini C, et al. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with post-operative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother Oncol. 2009;93:207–212. doi: 10.1016/j.radonc.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Perna L, Alongi F, Fiorino C, et al. Predictors of acute bowel toxicity in patients treated with IMRT whole pelvis irradiation after prostatectomy. Radiother Oncol. 2010;97:71–75. doi: 10.1016/j.radonc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Gay HA, Barthold HJ, O'Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour SK, Hashem SA, Bosch W, et al. Upper abdominal normal organ contouring guidelines and atlas: A Radiation Therapy Oncology Group consensus [e-pub ahead of print]. Pract Radiat Oncol. doi:10.1016/j.prro.2013.06.004, accessed January 17, 2017. [DOI] [PMC free article] [PubMed]
- 7.Dominello MM, Nalichowski A, Paximadis P, et al. Limitations of the bowel bag contouring technique in the definitive treatment of cervical cancer. Pract Radiat Oncol. 2014;4:e15–e20. doi: 10.1016/j.prro.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Hysing LB, Söhn M, Muren LP, Alber M. A coverage probability based method to estimate patient-specific small bowel planning volumes for use in radiotherapy. Radiother Oncol. 2011;100:407–411. doi: 10.1016/j.radonc.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 9.Tuomikoski L, Collan J, Keyrilinen J, Visap H, Saarilahti K, Tenhunen M. Adaptive radiotherapy in muscle invasive urinary bladder cancer—An effective method to reduce the irradiated bowel volume. Radiother Oncol. 2011;99:61–66. doi: 10.1016/j.radonc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 11.Ellis H, Logan BM, Dixon AK, Ellis H. Butterworth-Heinemann; Oxford, United Kingdom: 1999. Human Sectional Anatomy: Atlas of Body Sections, CT and MRI Images. [Google Scholar]
- 12.Gunnlaugsson A, Kjellén E, Nilsson P, Bendahl P-O, Willner J, Johnsson A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46:937–944. doi: 10.1080/02841860701317873. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee R, Chakraborty S, Nygren I, Sinha R. Small bowel dose parameters predicting grade ≥3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: An independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85:1225–1231. doi: 10.1016/j.ijrobp.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 14.Pollack A, Balogh A, Low D, et al. Radiation Therapy Oncology Group RTOG 0534: A Phase III Trial of Short Term Androgen Deprivation With Pelvic Lymph Node or Prostate Bed Only Radiotherapy (SPPORT) in Prostate Cancer Patients With a Rising PSA After Radical Prostatectomy. 2015.
- 15.Jhingran A, Portelance L, Miller B, Salehpour M. A Phase II Study of Intensity Modulated Radiation Therapy to the Pelvis for Postoperative Patients with Endometrial Carcinoma: Radiation Therapy Oncology Group Trial 0418. 2016. [DOI] [PubMed]
- 16.Muren LP, Hafslund R, Gustafsson A, Smaaland R, Dahl O. Partially wedged beams improve radiotherapy treatment of urinary bladder cancer. Radiother Oncol. 2001;59:21–30. doi: 10.1016/s0167-8140(00)00337-6. [DOI] [PubMed] [Google Scholar]
- 17.Roeske JC, Bonta D, Mell LK, Lujan AE, Mundt AJ. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–207. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Cavey ML, Bayouth JE, Colman M, Endres EJ, Sanguineti G. IMRT to escalate the dose to the prostate while treating the pelvic nodes. Strahlentherapie und Onkol. 2005;181:431–441. doi: 10.1007/s00066-005-1384-9. [DOI] [PubMed] [Google Scholar]
- 19.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price RA, Hannoun-Levi JM, Horwitz E, et al. Impact of pelvic nodal irradiation with intensity-modulated radiotherapy on treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:583–592. doi: 10.1016/j.ijrobp.2006.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.