Abstract
We operated on primary malignant melanoma of the lung, attaching the pericardium, diaphragm, and parietal pleura. A 48-year-old female was admitted to our hospital because of persistent dyspnea and cough. A preoperative computed tomography of the chest revealed 3 lesions in the right lung and a mass on the diaphragm between the right lung’s lower lobe and heart. A middle lobectomy was performed. The mass on the diaphragm had invaded the diaphragm and pericardium strictly. With a pericardiectomy and a diaphragmatic resection, the mass was removed in an en-bloc manner. Adjuvant chemotherapy was started 1 month after surgery and consisted of 5 days course of iv injection of cisplatin (90 mg/kg). The follow-up period was 5 years and uneventful. For primary pulmonary melanoma, even if it has intrapulmonary metastases, surgery and adjuvant chemotherapy can provide uneventful survival for more than 5 years.
Keywords: malign melanom, lung resection
Main Points
Pre- and postoperative images of primary pulmonary malign melanoma were analyzed.
A rare tumor in the lung was diagnosed by Allen and Drash criteria.
Surgery with chemotherapy caused the long-term survival.
Dyspnea and cough may be presentation symptoms.
Diaphragmatic and pericardial involvement does not exclude the surgery.
Introduction
Primary malignant melanoma of the lung is rare. Malignant melanoma occurs most often in the skin, but it can affect all areas comprising mucosal sites and organs such as the oral cavity, paranasal sinuses, esophagus, larynx, vagina, anorectal region, and liver. This refractory malignant tumor is rapidly increasing worldwide.1-4 Approximately 160 000 new cases of melanoma are diagnosed each year, and about 41 000 melanoma-related deaths occur annually.5 It comprises 1% of all malignant tumors and 3% of malignant tumors of the skin; primary malignant melanoma of the respiratory tract is the lowest, it accounts for 0.01% of all lung tumors.6-8 Because only a few cases have been described so far, the pathologic features, clinical behavior, and therapeutic options are not well-established.
This report presents the case of primary malignant melanoma of the lung, attaching pericardium, diaphragm, and parietal pleura. The aim of our study was to describe the importance of the diagnosis of primary pulmonary malignant melanoma through detailed examination, excluding any extrapulmonary origin, and emphasize the surgical specifications when the tumors were multiple and involved the surrounding structures.
According to the following criteria proposed by Allen and Drash, we concluded that the preoperative, intraoperative, and postoperative data proved that the tumor was primary lung melanoma with intrapulmonary metastasis: (1) no history suggestive of previous melanoma, (2) no demonstrable melanoma in any other organ at the time of surgery, (3) a solitary tumor in the surgical specimen from the lung, (4) tumor morphology compatible with that of a primary tumor, (5) no evidence of a primary melanoma elsewhere on autopsy, (6) obvious melanoma cells confirmed by immunohistochemical staining and possibly by electron microscopy for S-100 and HMB-45, (7) evidence of junctional change, and (9) invasion of the intact bronchial epithelium by melanoma cells.9-11
Case Presentation
A 48-year-old Turkish female was admitted to our hospital because of persistent dyspnea and cough. She has 15 healthy children. The patient had undergone a minor surgery for a chin abscess. Postoperative radiologic and positron emission tomographic (PET) examinations of the minor surgery site, including cranial computed tomography (CT), did not show any instances of malignant melanoma. Clinical examinations and routine laboratory tests showed no abnormality. A detailed history investigation revealed a chest x-ray taken 23 months before the thoracotomy that showed an undetected solitary pulmonary nodule.
A preoperative CT of the chest revealed 3 lesions in the right lung and a mass on the diaphragm between the right lung’s lower lobe and the heart (Figure 1A-E). Dimensions of the mass were 6 × 8 × 6 cm. Two of the lesions were in the middle lobe, and the third was in the upper lobe. The biggest one (3.6 × 3.2 × 3.6 cm) was in the middle lobe associated with surrounding atelectasis, and the lesion was lying on the parietal pleura. The inner part of the lesion had a low density. The preliminary diagnosis indicated an abscess or infected hydatid cysts. Within the same lobe, a lateral lesion, 1.4 × 1.3 cm, was observed with the same density and radiologic diagnosis. The upper lobe lesion, 2.4 × 1.3 cm, involved a minor fissure lying on the parietal pleura and had a soft tissue density. Multiple calcific and non-calcific lymph nodes were also followed in the mediastinal area. The suspected radiologic diagnosis was an infectious process. Common carotid arteries were separately arising from ascending aorta. Truncus brachiocephalicus was absent. Aorta was at the right paravertebral region. As the 2 pleural biopsies in chest disease show benign cytology and suspected infection process radiologically, genetic study (including BRAF mutation) has not been done. Since the periferal location, the bronchoscopic examination has not been performed.
Figure 1.
(A) Two middle lob lesions. (B) Mass on the diaphragm between right lower lobe and heart. (C) Axial CT image showing middle lobe tumors. (D) Axial CT image showing middle and upper lobe tumors. (E) Axial CT image showing the mass on the diaphragm anteriorly. CT, computed tomography.
After informed consent has taken for operation, a thoracotomy was done with a posterolateral incision, and middle lobectomy was performed. Two of the 3 lung tumors were within the middle lobe. Upper lobe tumor was lying on the pleura and it was removed together with the pleura with the precision excision technique. The mass on the diaphragm had invaded the diaphragm and pericardium strictly, but as it was weakly attached to the lower lobe, it was easily dissected from the lobe. With pericardiectomy and the diaphragmatic resection, the mass was removed in an en-bloc manner. The diaphragmatic defect was closed primarily. A patch was sewn on the pericardial defect. Lymph nodes found in the operative area were removed, and lymph node dissection has not been performed. A histopathologic diagnosis of the samples indicated malign melanoma. Pathologic examination showed that there were 2 broad nests of atypical melanocyte cells at the periphery of the lung. There were atypical mitotic figures and irregular and broad necrosis. In particular, melanin pigment was irregularly dispersed around the tumor and medium-density lymphocytes were also observed in the tumor (Figure 2A-D). Both frozen sections and postoperative histologic evaluation of the lung and pleura samples detected tumor-free resection margins.
Figure 2.
(A) Low-power view shows a broad proliferation and necrosis of large atypical melanocytes ×10. (B) High-power view shows necrosis, atypical melanocytes, and irregularly dispersed melanin pigment ×40. (C) Immuno-expression of CD68 in tumor cells (CD68, ×40). (D) Immuno-expression of HMB45 in tumor cells (HMB45, ×40).
Detailed dermatologic and ophthalmologic examinations revealed no evidence of cutaneous or ocular primary melanoma. Additionally, gastrointestinal endoscopy, colonoscopy, endoscopy of the nasal cavity, and PET scans of the brain were performed. They proved no evidence of malignant melanoma elsewhere than the lung.
Adjuvant chemotherapy was started 1 month after surgery and consisted of 5 days of iv injection of cisplatin (90 mg/kg). The injections were repeated every 4 weeks, 6 times. Temozolomide (225 mg/day, per oz) was prescribed for 6 months. Postchemotherapy P-A was normal (Figure 3). Two years after the operation, she is still alive and healthy (Figure 4). Up to the fifth year, any metastasis has not been detected with control CT, and laboratory tests were done 2 times. At end of the fifth year, control CT showed a heterogeneous mass lesion in the operation side of the lung, and the chemotherapy regimen was repeated. During oncologic treatment, severe pneumonia has developed. Seven years after the operation, the patient died due to oncologic reasons and pneumonia.
Figure 3.

Postchemotherapy P-A x-ray, 6 months after the operation.
Figure 4.
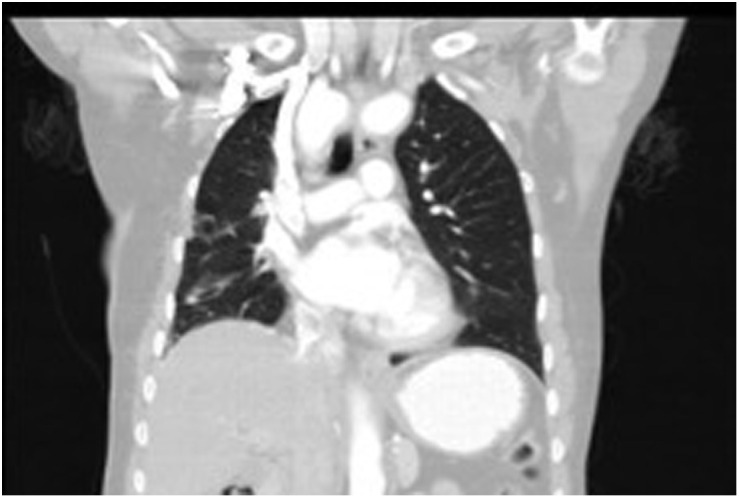
Control CT 2 years after the operation. CT, computed tomography.
Discussion
The extrapulmonary origin of malignant melanoma must be excluded by detailed examination to establish the diagnosis of primary malignant melanoma as we did.12
Approximately 5-10% of patients with metastatic melanoma have a primary melanoma of unknown origin.13,14 The involvement of multiple nodules of the lung is generally considered intrapulmonary metastases. Our patient has intrapulmonary, pericardial, and diaphragmatic metastases. It has been shown that it could have intrapulmonary and intracranial metastases.14,15 There were multiple lesions in our case, so we had to determine whether the tumor was a primary or secondary lesion.
Primary cases suggest that melanoma can arise in the lung as a primary tumor, probably from residual melanoblasts. This tumor is frequently endobronchial and often manifests with symptoms of cough, hemoptysis, and lobar collapse.14 Cutaneous squamous cell carcinoma metastatic to the lung, initially diagnosed as a lung primary, but whose diagnosis was later clarified as metastasis from prior skin primary by the presence of a strong UV (ultraviolet) radiation mutational signature.16 This signature is strongly associated with tumors arising from UV-exposed skin, signature analysis can serve as a helpful diagnostic tool to determine the etiology and site of origin of tumors.13 Although the tumor should be removed surgically, whether it occurs as a single lesion or multiple lesions, aggressive surgical resection, irrespective of lymph node involvement, offers possible long-term survival in some patients. According to case report studies about BRAF mutation, surgical treatment offers the only curative option for the patients having BRAF mutation, but when the disease is unresectable or negative margins cannot be achieved, immunotherapy and targeted therapy (anti-PD1, pembrolizumab) is a valuable option to obtain disease control.1
The optimal treatment for patients with primary malignant melanoma of the lung remains to be determined.11,17 Some studies have demonstrated that the prognosis for surgically resected patients is better than that of non-surgically treated patients.13,14 In our case, because of diaphragmatic and pericardial involvement, an open surgery was performed. Resection should be done with clear margins and should be complete. After pneumonectomy, there was a disease-free survival for 60 months.7 Our patient has also a 5-year disease-free period and lived 2 more years with recurrence and chemotherapy. On the other hand, the patients who refused surgical treatment can have only limited survival (even 2 months).18
In conclusion, primary malignant pulmonary melanoma, even if it has intrathoracic metastases, can be successfully treated surgically.
Footnotes
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.E.B.; Design – A.E.B.; Data Collection and/or Processing – M.K.; Analysis and/or Interpretation – A.M.; Literature Search – M.K.; Writing Manuscript – A.E.B., İ.Ç.; Critical Review – İ.Ç.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Bernal L, Restrepo J, Alarcón ML.et al. Primary BRAF mutant melanoma of the lung treated with immunotherapy and pulmonary bilobectomy: a case report. Am J Case Rep. 2021;22:e927757 -e927757-6. 10.12659/AJCR.927757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbas O, Miller DD, Bhawan J. Cutaneous malignant melanoma: update on diagnostic and prognostic biomarkers. Am J Dermatopathol. 2014;36(5):363 379. 10.1097/DAD.0b013e31828a2ec5) [DOI] [PubMed] [Google Scholar]
- 3. Deng S, Sun X, Zhu Z.et al. BMC Pulm Med. 2002;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paliogiannis P, Fara AM, Pintus G.et al. Primary malignant melanoma of the lung: a case report and literature review. Medicina. 2020;56(11):576. 10.3390/medicina56110576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74 108. 10.3322/canjclin.55.2.74) [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Bing Z, Xu X, Cui Y. Primary pulmonary malignant melanoma: case report and literature review. Thorac Cancer. 2018;9(9):1185 1189. 10.1111/1759-7714.12798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagwell SP, Flynn SD, Cox PM, Davison JA. Primary malignant melanoma of the lung. Am Rev Respir Dis. 1989;139(6):1543 1547. 10.1164/ajrccm/139.6.1543) [DOI] [PubMed] [Google Scholar]
- 8. Mahowald MK, Aswad BI, Okereke IC, Ng T. Long-term survival after pneumonectomy for primary pulmonary malignant melanoma. Ann Thorac Surg. 2015;99(4):1428 1430. 10.1016/j.athoracsur.2014.06.110) [DOI] [PubMed] [Google Scholar]
- 9. Scolyer RA, Bishop JF, Thompson JF. Primary Melanoma of the lung. In: Raghavan D, Brecher ML, Johnson DH.et al., eds. Textbook of Uncommon Cancer. 3rd ed. West Sussex: John Wiley & Sons Ltd; 2006:293 298. [Google Scholar]
- 10. Jensen OA, Egedorf J. Primary malignant melanoma of the lung. Scand J Respir Dis. 1967;48(2):127 135. [PubMed] [Google Scholar]
- 11. Allen MS, Drash EC. Primary melanoma of the lung. Cancer. 1968;21(1):154 159. [DOI] [PubMed] [Google Scholar]
- 12. Ost D, Joseph C, Sogoloff H, Menezes G. Primary pulmonary melanoma: case report and literature review. Mayo Clin Proc. 1999;74(1):62 66. 10.4065/74.1.62) [DOI] [PubMed] [Google Scholar]
- 13. Serna MJ, Vázquez-Doval J, Sola MA, Ruiz de Erenchun F, Quintanilla E. Metastatic melanoma of unknown primary tumor. Cutis. 1994;53(6):305 308. [PubMed] [Google Scholar]
- 14. Yang C, Sanchez-Vega F, Chang JC.et al. Lung-only melanoma: UV mutational signature supports origin from occult cutaneous primaries and argues against the concept of primary pulmonary melanoma. Mod Pathol. 2020;33(11):2244 2255. 10.1038/s41379-020-0594-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seitelman E, Donenfeld P, Kay K, Takabe K, Andaz S, Fox S. Successful treatment of primary pulmonary melanoma. J Thorac Dis. 2011;3(3):207 208. 10.3978/j.issn.2072-1439.2011.04.02) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sholl LM, Do K, Shivdasani P.et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1(19):e87062. 10.1172/jci.insight.87062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong L, Liu XY, Zhang WD.et al. Primary pulmonary malignant melanoma: a clinicopathologic study of two cases. Diagn Pathol. 2012;7(23):123. 10.1186/1746-1596-7-123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agrawal CR, Talwar V, Tayal J, Babu Koyyala VPB, Goyal P. Primary pulmonary melanoma: an unexpected diagnosis. Indian J Pathol Microbiol. 2018;61(4):636 638. 10.4103/IJPM.IJPM_490_17) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a 
