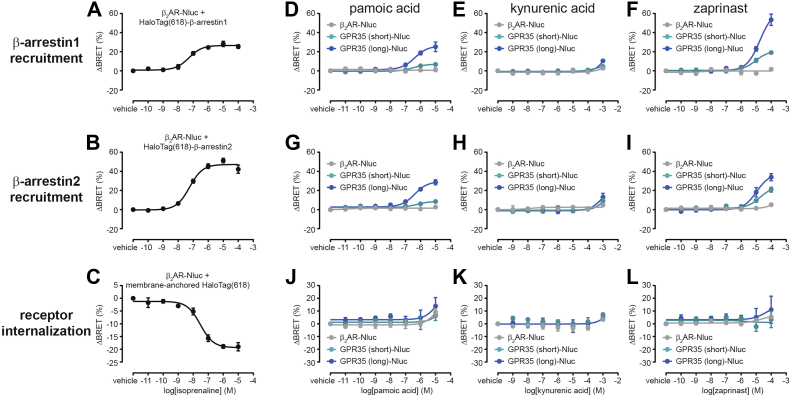
Figure 6.
GPR35–β-arrestin interaction and receptor internalization.A and B, recruitment of HaloTag-β-arrestin1 (A) or of HaloTag-β-arrestin2 (B) to isoprenaline-stimulated β2AR-Nluc. C, internalization of isoprenaline-stimulated β2AR-Nluc. D–F, recruitment of HaloTag-β-arrestin1 to pamoic acid- (D), kynurenic acid- (E), or zaprinast-stimulated (F) β2AR-, GPR35 short-, or GPR35 long-Nluc. G–I, recruitment of HaloTag-β-arrestin2 to pamoic acid- (G), kynurenic acid- (H), or zaprinast-stimulated (I) β2AR-, GPR35 short-, or GPR35 long-Nluc. J–L, internalization of pamoic acid- (J), kynurenic acid- (K), or zaprinast-stimulated (L) β2AR-, GPR35 short-, or GPR35 long-Nluc. Data show mean ± s.e.m. of three to four independent experiments conducted in transiently transfected HEK293A cells. Vehicle-treated ΔBRET values were set to 0. BRET, bioluminescence resonance energy transfer; HEK293, human embryonic kidney 293A; Nluc, NanoLuciferase.