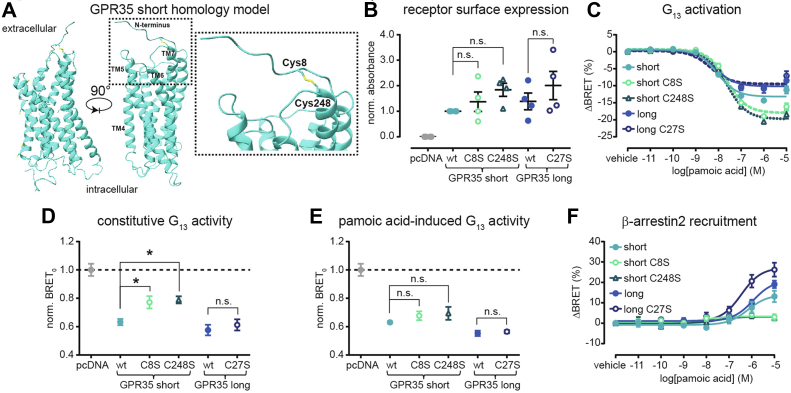
Figure 7.
Extracellular cysteine residues in GPR35 isoforms regulate constitutive and ligand-induced receptor-transducer coupling.A, homology model of GPR35 short indicating the formation of a disulfide bridge between Cys8 in the N-terminus and Cys248 in ECL3. B, surface expression levels of the indicated GPR35 isoform mutants and WT. The negative control (pcDNA transfection) was normalized to 0, and the expression of GPR35 short was set to 1.0 for each individual experiment. C, Pamoic acid–induced activation of the G13 activation mediated by the indicated GPR35 isoform mutants and WT. D and E, BRET0 analysis of G13 activity prior (D) and after (E) agonist-induced GPR35 activation. F, recruitment of β-arrestin2 to pamoic acid–stimulated GPR35 isoform mutants and WT. Data show mean ± s.e.m. of four to five independent experiments conducted in transiently transfected HEK293A cells. Statistical significance in (B), (D), and (E) was tested using one-way ANOVA followed by Tukey’s multiple comparison; p < 0.5. BRET, bioluminescence resonance energy transfer; HEK293, human embryonic kidney 293A.