Abstract
Pregnant persons are at higher risk of severe COVID-19. Although vaccination is recommended, COVID-19 vaccination rates are lower among pregnant persons compared to the non-pregnant population. We aimed to evaluate acceptance of any dose of COVID-19 vaccine during pregnancy. A national online cross-sectional survey of US adults who were pregnant between December 2020 and July 2021 was used to measure COVID-19 vaccine behaviors, attitudes, and beliefs. Post-stratification weighting was used to ensure representativeness to the US population. Marginal log-binomial models were used to estimate adjusted prevalence ratios (aPR) of COVID-19 vaccine acceptance, accounting for sociodemographic factors. Of 5,660 who responded to survey advertisements, 2,213 met eligibility criteria and completed the survey; 55.4% of respondents received or planned to receive COVID-19 vaccine prior to or during pregnancy, 27.0% planned to vaccinate after pregnancy, 8.8% were unsure and 8.7% had no plans to vaccinate. Individuals were more likely to receive or plan to receive COVID-19 vaccine if they had group prenatal care (aPR 1.57; 95% CI 1.40, 1.75), were employed in a workplace with a policy recommending vaccination (aPR 1.15; 95% CI 1.06, 1.26), and believed COVID-19 vaccines are safe (aPR 2.86; 95% CI 2.49, 3.29). Pregnant persons who were recommended COVID-19 vaccination by their healthcare provider less commonly reported concerns about vaccine safety (35.5% vs 55.9%) and were more likely to accept COVID-19 vaccines (aPR 1.52; 95% CI 1.31, 1.76). COVID-19 vaccine acceptance during pregnancy is not universal and public health intervention will be needed to continue to increase vaccine coverage.
Keywords: COVID-19, Maternal vaccination, Pregnancy, Vaccine acceptance
1. Introduction
Pregnant persons are more likely than non-pregnant persons to experience severe COVID-19, including higher rates of admission to intensive care unit and requirement for invasive mechanical ventilation or extracorporeal membrane oxygenation (Allotey et al., 2020, Ko et al., 20212021, Vousden et al., 2021, Zambrano et al., 2020). Recent data suggest the severity of COVID-19 during pregnancy may have increased following the dominance of the Delta B.1.617.2 virus (Adhikari et al., 2022). Physiological changes during pregnancy impact the immune system which increases the severity of infectious illnesses subsequently increasing maternal and fetal morbidity and mortality (Kourtis et al., 2014). As a result, pregnant and recently pregnant persons are considered a high-risk group for COVID-19 by the US Centers for Disease Control and Prevention (CDC) (CDC, 2021) In addition to the direct health effects on the mother, adverse health outcomes have been observed for infants born to SARS-CoV-2 infected mothers, including preterm birth, cesarean delivery, postpartum hemorrhage, and admission to neonatal intensive care (Allotey et al., 2020, Blitz et al., 2021, Huntley et al., 2021, Oltean et al., 2021).
In December 2020, the American College of Obstetricians and Gynecologists (ACOG) and the CDC recommended that pregnant and lactating individuals should be given access to COVID-19 vaccines (CDC, 2021, ACOG, 2020). Despite this, vaccination rates are low around the time of pregnancy and there is evidence to show that individuals delay vaccination around the time of pregnancy (Razzaghi et al., 2021, Razzaghi et al., 2022). A recent analysis of National Immunization Survey (COVID-19 supplemental module) data showed that among women aged 18–49 years old, COVID-19 vaccination coverage is lower among pregnant individuals (45 %), those who are trying to become pregnant (49 %) and those breastfeeding (51 %) compared to non-pregnant women and those not trying to conceive (65 %) (Razzaghi et al., 2022). Given the higher rate of serious complications observed among pregnant persons with COVID-19 (Zambrano et al., 2020, Kasehagen et al., 2021, DeSisto et al., 2021), further understanding of the factors associated with vaccine acceptance, delay and refusal around the time of pregnancy is needed.
2. Methods
2.1. Study sample
Between May and July 2021, we conducted a national, online cross-sectional survey of pregnant or recently pregnant US adults. Individuals were invited to participate through internet advertising that targeted adults based on search history and social media group membership indicative of pregnancy or the presence of a newborn in the household. Individuals were eligible to participate if they were 18 to 45 years of age, resident in the US, and had been pregnant any time since January 2020 (i.e., beginning of SARS-CoV-2 activity in the US). For this analysis, we restricted the sample to those who had a pregnancy ending after December 2020 (i.e., period of COVID-19 vaccine availability in the US). Quota-based sampling based on age, race/ethnicity and US region of residence was used to promote representativeness of the survey results. Based on a population size of 3.7 million U.S. births nationally, 2,400 participants were needed to estimate COVID-19 vaccination with a margin of error ±2 % and 95 % confidence level. The study was reviewed and approved by the University of San Francisco Institutional Review Board.
2.2. Measures
Following provision of informed consent, respondents were asked to complete a 30-minute online survey (Appendix). The survey included items on pregnancy history, prenatal care, social support, risk behaviors including substance use, experiences with COVID-19 infection and vaccines, health-related quality-of-life, and sociodemographic information and zip code of residence. Where available, survey items were adapted from the US CDC’s Pregnancy Risk Assessment Monitoring System. Zip codes were used to determine rurality of residence based on 2013 NCHS urban–rural codes (Ingram and Franco, 2014). Respondents were asked to self-report information on the receipt of COVID-19 vaccines. Those who reported receiving ≥1 dose of COVID-19 vaccine were asked whether they received the vaccine before, during or after pregnancy (for postpartum participants) and reasons for receiving COVID-19 vaccine (multiple reasons could be provided). Those who reported not receiving any COVID-19 vaccine were asked about their plans to receive COVID-19 vaccine. Those with no plans to vaccinate were asked about their reasons for not receiving COVID-19 vaccine. Participants were additionally asked 10 items regarding their attitudes and perceptions of COVID-19 vaccines, and 8 items regarding their attitudes and perceptions of other vaccines. Survey items asked respondents to report on a 5-point Likert scale how well they agreed with each vaccine-related item (“strongly disagree”, “disagree”, “neither disagree nor agree”, “agree” or “strongly agree”).
Based on survey responses, participants were categorized into four mutually exclusive groups: 1) those who received or plan to receive COVID-19 vaccine before or during pregnancy (COVID-19 vaccine acceptance); 2) those who plan to receive COVID-19 vaccine after pregnancy (COVID-19 vaccine delay); 3) those who were uncertain whether they would receive a COVID-19 vaccine (COVID-19 vaccine hesitancy); and 4) those who do not plan to receive a COVID-19 vaccine (COVID-19 vaccine refusal).
2.3. Statistical analysis
We applied post-stratification weights to the sample data by maternal age, race/ethnicity, and US census region of residence. Weights were calculated based on US natality data for 2016–2019 (Table S1). We estimated weighted percentages and 95 % confidence intervals for all study outcomes. We compared respondent characteristics and self-reported attitudes toward vaccines by vaccination group using Chi-squared tests and examining overlap between confidence intervals. To assess pregnancy, clinical, employment and attitudinal factors associated with COVID-19 vaccine acceptance, we used marginal log-binomial models to estimate prevalence ratios and 95 % confidence intervals. For log-binomial models, we defined COVID-19 vaccine acceptance as having received ≥ 1 dose of COVID-19 vaccine before or during pregnancy or planning to receive vaccine during pregnancy. Those delaying, expressing hesitancy or planning to refuse COVID-19 vaccines formed the comparator. Models allowed for clustering within state and adjusting for maternal age, race/ethnicity, birth overseas, rurality and region of residence, educational attainment, and insurance status. Because <6 % of variables were missing data, we performed a complete case analysis.
3. Results
In total, 6,089 individuals responded to the study advertisement. Of these, 429 (7.0 %) were screened ineligible. Of the 5,660 eligible participants, 687 (12.1 %) did not consent to participate (Figure S1). Of the 4,973 participants who consented to participate, 3,392 (68.2 %) completed the survey (Figure S2), and 2,360 (69.6 %) of these respondents had a pregnancy during the period of vaccine availability in the US. We excluded 147 participants who did not provide complete information on COVID-19 vaccination, leaving 2,213 participants for inclusion in the final analysis. The characteristics of respondents are provided in Table S1. The weighted sample characteristics were similar to those of the US population of births in terms of maternal age, race/ethnicity, US Census region of residence, and rurality of residence (Table S1).
Overall, 55.4 % (95 % CI 53.3, 57.6 %) of respondents had received or planned to receive ≥1 dose of COVID-19 vaccine during pregnancy (8.4 % received prior to pregnancy, 45.3 % received during pregnancy, and 1.7 % planned to receive during pregnancy); 27.0 % (95 % CI 25.1, 28.9 %) planned to wait until after pregnancy; 8.8 % (95 % CI 7.6, 10.1 %) were unsure whether they would receive a COVID-19 vaccine; and 8.7 % (95 % CI 7.5, 10.0 %) had no plans to receive a COVID-19 vaccine (Table 1). Those who delayed or refused vaccination were more commonly 18–24 years of age, born overseas, and had lower educational attainment and household income (P < 0.001). Those who delayed or were hesitant toward COVID-19 vaccination were more commonly Latina/x compared to those who accepted or refused COVID-19 vaccination. Those who delayed vaccination commonly reported receiving other recommended vaccines (influenza: 63.9 %; Tdap: 68.5 %). Few respondents who refused COVID-19 vaccination reported receiving other recommended vaccines (influenza: 15.4 %; Tdap: 30.2 %) (Table 1).
Table 1.
Characteristics of pregnant persons, by COVID-19 vaccine acceptance – United States, May to July 2021.
| Characteristic | COVID-19 vaccine acceptance* (n = 1,238) |
COVID-19 vaccine delay (n = 591) |
COVID-19 vaccine hesitancy (n = 192) |
COVID-19 vaccine refusal (n = 192) |
|---|---|---|---|---|
| n (weighted%) | n (weighted%) | n (weighted%) | n (weighted%) | |
| Maternal age† | ||||
| 18–24 years | 79 (6.5 %) | 80 (12.2 %) | 41 (20.7 %) | 59 (28.9 %) |
| 25–29 years | 390 (30.9 %) | 164 (27.6 %) | 53 (26.5 %) | 68 (35.7 %) |
| 30–34 years | 450 (36.2 %) | 187 (31.5 %) | 51 (27.2 %) | 39 (20.6 %) |
| 35–39 years | 261 (21.5 %) | 134 (23.9 %) | 35 (18.9 %) | 24 (13.5 %) |
| ≥40 years | 58 (4.9 %) | 26 (4.9 %) | 12 (6.7 %) | 2 (1.3 %) |
| Race/ethnicity† | ||||
| White, Non-Hispanic | 896 (74.6 %) | 459 (79.7 %) | 144 (74.6 %) | 161 (84.4 %) |
| Black, Non-Hispanic | 127 (9.0 %) | 12 (2.2 %) | 8 (5.5 %) | 9 (4.4 %) |
| Hispanic or Latina/x | 102 (8.1 %) | 98 (15.0 %) | 33 (16.6 %) | 18 (8.8 %) |
| Pacific Islander | 0 (0 %) | 0 (0 %) | 1 (0.5 %) | 0 (0 %) |
| American Indian / Alaskan Native | 5 (0.5 %) | 4 (0.6 %) | 2 (1.3 %) | 2 (1.5 %) |
| Asian | 104 (7.5 %) | 16 (2.2 %) | 2 (0.7 %) | 2 (0.9 %) |
| Multiple Races | 4 (0.3 %) | 2 (0.3 %) | 2 (0.9 %) | 0 (0 %) |
| Born overseas† | ||||
| Yes | 105 (8.5 %) | 86 (13.9 %) | 25 (13.4 %) | 9 (4.5 %) |
| No | 1,119 (91.5 %) | 496 (86.1 %) | 163 (86.6 %) | 178 (95.5 %) |
| Educational attainment† | ||||
| High school diploma or less | 114 (9.1 %) | 66 (10.1 %) | 33 (17.1 %) | 32 (16.0 %) |
| Some college or technical school | 163 (13.1 %) | 102 (17.6 %) | 48 (26.6 %) | 65 (36.1 %) |
| College degree | 428 (35.0 %) | 213 (36.4 %) | 68 (33.8 %) | 67 (35.0 %) |
| Graduate degree | 533 (42.9 %) | 210 (35.9 %) | 42 (22.4 %) | 26 (12.8 %) |
| Household income† | ||||
| <$20,000 | 39 (3.4 %) | 42 (6.2 %) | 21 (10.6 %) | 23 (11.2 %) |
| $20,000 to $34,999 | 129 (10.3 %) | 64 (10.2 %) | 30 (16.8 %) | 29 (14.3 %) |
| $35,000 to $49,999 | 120 (9.3 %) | 58 (9.2 %) | 25 (12.7 %) | 27 (15.3 %) |
| $50,000 to $74,999 | 195 (15.9 %) | 111 (19.6 %) | 34 (18.0 %) | 42 (23.0 %) |
| $75,000 to $99,999 | 297 (24.1 %) | 127 (21.9 %) | 41 (20.8 %) | 40 (20.5 %) |
| $100,000 to $149,999 | 281 (22.8 %) | 128 (22.3 %) | 25 (12.6 %) | 23 (11.5 %) |
| ≥$150,000 | 177 (14.2 %) | 61 (10.6 %) | 16 (8.5 %) | 8 (4.1 %) |
| Insurance coverage† | ||||
| Private health insurance | 1,059 (85.5 %) | 457 (79.0 %) | 127 (65.4 %) | 132 (67.4 %) |
| Medicaid | 120 (9.7 %) | 88 (14.1 %) | 31 (16.8 %) | 38 (20.9 %) |
| Other health insurance | 47 (3.8 %) | 29 (4.4 %) | 20 (10.0 %) | 15 (8.0 %) |
| Uninsured | 12 (1.0 %) | 17 (2.4 %) | 14 (7.8 %) | 7 (3.8 %) |
| Break in insurance during pregnancy | ||||
| Yes | 50 (3.8 %) | 30 (4.9 %) | 12 (6.7 %) | 3 (1.8 %) |
| No | 1,188 (96.2 %) | 561 (95.1 %) | 180 (93.3 %) | 189 (98.2 %) |
| Residence§ | ||||
| Large Central Metro | 307 (24.4 %) | 138 (22.2 %) | 33 (16.7 %) | 33 (16.2 %) |
| Large Fringe Metro | 257 (21.2 %) | 129 (21.6 %) | 35 (17.4 %) | 37 (18.4 %) |
| Medium Metro | 299 (24.2 %) | 171 (29.2 %) | 60 (31.5 %) | 55 (28.8 %) |
| Small Metro | 144 (11.7 %) | 68 (11.9 %) | 34 (16.6 %) | 28 (15.2 %) |
| Micropolitan | 125 (10.0 %) | 58 (10.0 %) | 18 (10.8 %) | 27 (14.8 %) |
| Non-Core | 106 (8.3 %) | 27 (5.0 %) | 12 (7.0 %) | 12 (6.5 %) |
| Region§ | ||||
| Northeast | 204 (16.1 %) | 81 (13.5 %) | 28 (14.3 %) | 20 (10.5 %) |
| South | 433 (35.4 %) | 216 (36.7 %) | 82 (42.8 %) | 84 (44.8 %) |
| Midwest | 319 (21.9 %) | 147 (26.0 %) | 38 (20.8 %) | 46 (24.0 %) |
| West | 276 (21.9 %) | 147 (23.7 %) | 44 (22.1 %) | 41 (20.1 %) |
| US territory | 6 (0.5 %) | 0 (0 %) | 0 (0 %) | 1 (0.6 %) |
| Employment† | ||||
| Employed | 1,004 (80.7 %) | 371 (64.8 %) | 102 (53.3 %) | 106 (53.2 %) |
| Essential worker† | 586 (47.4 %) | 221 (39.1 %) | 77 (40.8 %) | 68 (34.8 %) |
| Essential HCP† | 285 (23.3 %) | 108 (18.9 %) | 40 (21.1 %) | 25 (12.8 %) |
| Essential HCP with patient contact§ | 259 (21.0 %) | 102 (18.0 %) | 37 (19.5 %) | 22 (11.3 %) |
| Military§ | 36 (2.4 %) | 5 (0.7 %) | 4 (1.8 %) | 1 (0.6 %) |
| Unemployed | 182 (15.0 %) | 168 (26.8 %) | 71 (36.5 %) | 74 (40.6 %) |
| Homemaker, Student or Unable to work | 52 (4.3 %) | 52 (8.3 %) | 19 (10.2 %) | 12 (6.2 %) |
| Workplace recommended vaccination§ | ||||
| Yes | 704 (55.8 %) | 218 (38.5 %) | 62 (32.9 %) | 50 (25.5 %) |
| No | 534 (44.2 %) | 373 (61.5 %) | 130 (67.1 %) | 142 (74.5 %) |
| Pre-existing medical conditions | ||||
| Diabetes | 28 (2.1 %) | 13 (2.5 %) | 5 (3.1 %) | 3 (1.4 %) |
| Chronic hypertension | 40 (3.6 %) | 24 (5.0 %) | 10 (5.7 %) | 11 (6.1 %) |
| Depression | 147 (12.9 %) | 95 (16.3 %) | 30 (16.7 %) | 29 (15.1 %) |
| Asthma | 113 (9.8 %) | 57 (10.1 %) | 21 (11.6 %) | 22 (11.4 %) |
| Coronary heart disease | 12 (0.8 %) | 2 (0.4 %) | 0 (0 %) | 1 (<0.1 %) |
| Obesity† | 171 (14.2 %) | 125 (21.5 %) | 39 (21.1 %) | 37 (18.7 %) |
| PNC Provider† | ||||
| Private obstetrician | 760 (62.4 %) | 407 (69.8 %) | 121 (64.8 %) | 106 (56.4 %) |
| Primary care provider | 110 (8.6 %) | 19 (2.9 %) | 9 (4.9 %) | 7 (4.0 %) |
| Nurse or midwife | 338 (26.5 %) | 141 (23.4 %) | 53 (25.5 %) | 69 (34.5 %) |
| Government clinic | 3 (0.3 %) | 9 (1.3 %) | 6 (2.9 %) | 4 (1.7 %) |
| Other provider | 27 (2.2 %) | 15 (2.6 %) | 3 (1.9 %) | 6 (3.3 %) |
| Received group PNC† | ||||
| Yes | 297 (21.6 %) | 28 (4.5 %) | 7 (4.1 %) | 10 (4.9 %) |
| No | 941 (78.3 %) | 563 (95.5 %) | 192 (95.9 %) | 182 (95.1 %) |
| HCP Recommended vaccination | ||||
| Yes | 790 (65.3 %) | 361 (61.7 %) | 72 (37.9 %) | 52 (26.8 %) |
| No | 448 (34.7 %) | 230 (38.3 %) | 120 (62.1 %) | 140 (73.1 %) |
| Parity† | ||||
| Parity 0 | 408 (32.5 %) | 117 (19.4 %) | 35 (19.0 %) | 78 (39.6 %) |
| Parity 1 | 531 (42.3 %) | 249 (42.3 %) | 68 (35.1 %) | 65 (34.2 %) |
| Parity ≥ 2 | 299 (25.2 %) | 225 (38.3 %) | 89 (45.8 %) | 49 (26.2 %) |
| Calendar time of conception | ||||
| Prior to Sept 2020 | 264 (21.7) | 326 (55.6) | 85 (42.7) | 5 (1.7) |
| Sept 2020–Dec 2020 | 454 (37.1) | 185 (30.7) | 76 (41.1) | 110 (57.5) |
| After Dec 2020 | 520 (41.1) | 80 (13.6) | 31 (16.2) | 77 (40.8) |
| Pregnancy Intention⁋ | ||||
| Intended | 908 (73.5 %) | 416 (70.9 %) | 119 (61.3 %) | 125 (66.0 %) |
| Unintended | 330 (26.4 %) | 175 (29.1 %) | 73 (38.7 %) | 67 (34.0 %) |
| Marital status† | ||||
| Married or member of unmarried couple | 1,144 (91.9 %) | 496 (83.8 %) | 141 (75.6 %) | 155 (81.9 %) |
| Unmarried | 94 (8.1 %) | 95 (16.2 %) | 51 (27.4 %) | 37 (18.1 %) |
| Sexual orientation | ||||
| Lesbian or gay | 11 (0.7 %) | 6 (1.1 %) | 0 (0 %) | 2 (1.2 %) |
| Heterosexual | 1,122 (90.4 %) | 527 (89.0 %) | 182 (94.3 %) | 176 (92.5 %) |
| Bisexual | 90 (7.7 %) | 48 (8.3 %) | 7 (4.4 %) | 11 (5.3 %) |
| Something else | 15 (1.2 %) | 10 (1.6 %) | 3 (1.3 %) | 3 (1.0 %) |
| Received other recommended vaccines | ||||
| Influenza vaccine† | 509 (41.8 %) | 378 (63.9 %) | 91 (46.2 %) | 32 (15.4 %) |
| Tdap vaccine† | 552 (45.3 %) | 399 (68.5 %) | 105 (54.9 %) | 56 (30.2 %) |
| Diagnosed with COVID-19* | ||||
| Yes | 2 (0.1 %) | 2 (0.4 %) | 1 (0.6 %) | 1 (0.3 %) |
| No, but think they had COVID-19 | 130 (10.7 %) | 117 (20.4 %) | 32 (16.7 %) | 62 (33.7 %) |
| No | 1,101 (88.9 %) | 464 (79.2 %) | 157 (82.7 %) | 126 (65.9 %) |
| Hospitalized with COVID-19 | ||||
| Yes | 37 (2.7 %) | 11 (2.0 %) | 3 (1.9 %) | 4 (2.0 %) |
| No | 1,201 (97.3 %) | 580 (98.0 %) | 189 (98.1 %) | 188 (97.9 %) |
Abbreviations: HCP, healthcare personnel; Tdap, tetanus-diphtheria-acellular pertussis.
*COVID-19 vaccine acceptance was defined as those who had received the vaccine prior to or during pregnancy or planned to receive the vaccine during pregnancy; COVID-19 vaccine delay was defined as those who planned to receive COVID-19 vaccine, but after pregnancy; COVID-19 vaccine hesitancy was defined as those who were uncertain whether they would receive a COVID-19 vaccine; COVID-19 vaccine refusal was defined as those who had no plans to receive COVID-19 vaccine.
Chi-squared comparison significant at P < 0.001.
Chi-squared comparison significant a P < 0.05.
Chi-squared comparison significant at P < 0.01.
3.1. Reasons for COVID-19 vaccine acceptance or refusal
Among vaccinated respondents and those planning to receive a COVID-19 vaccine, 34.2 % reported their main reason for vaccination was wanting to protect themselves; 32.7 % reported wanting to protect their baby; 18.6 % wanted to protect their community; 4.2 % reported being required to receive the COVID-19 vaccine for their work or school; 2.1 % reported other reasons for COVID-19 vaccination. Among those who refused COVID-19 vaccine, the most common reason provided was concerns about vaccine side effects (42.6 %); 22.6 % of unvaccinated respondents believed the vaccine was not necessary; 6.2 % said their plans to vaccinate could change after breastfeeding; and 4.5 % were unsure whether the vaccine would work. No respondent reported barriers to accessing COVID-19 vaccines (i.e., uncertain how to access, affordability) as a reason for not receiving a COVID-19 vaccine.
3.2. COVID-19 vaccine attitudes
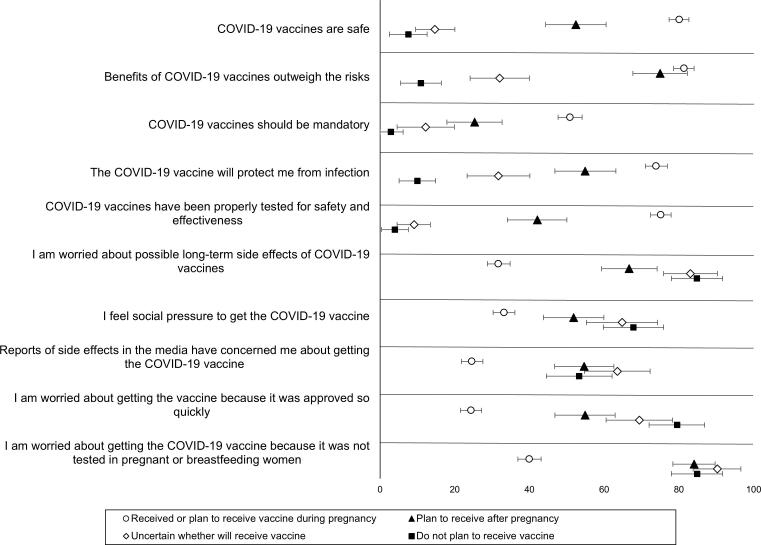
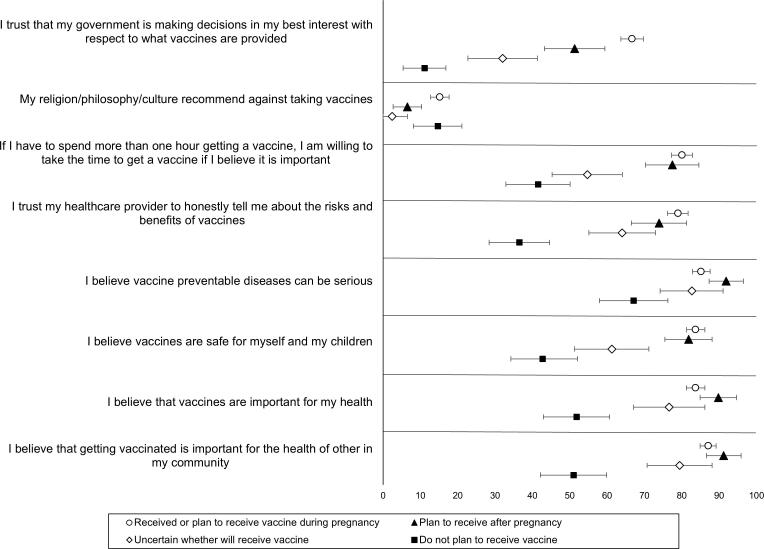
A higher proportion of pregnant persons who were vaccinated or planning to vaccinate supported the belief that COVID-19 vaccines are safe, that the benefits outweigh the risks, and that the vaccine would protect them against COVID-19 (Fig. 1). When we examined attitudes toward other vaccines, the primary difference between those who accepted COVID-19 vaccines and those who did not was in relation to trust in government: 67 % of those vaccinated or planning to vaccinate reported that they trusted their government to make vaccine decisions in their best interest, whereas 51 % of those who planned to delay vaccination until after pregnancy, 32 % of those who were unsure and 11 % of those who refused vaccination endorsed this belief (Fig. 2).
Fig. 1.
Attitudes toward COVID-19 vaccines expressed by pregnant persons, by COVID-19 vaccine acceptance – United States, May to July 2021.
Fig. 2.
Attitudes toward general vaccines expressed by pregnant persons, by COVID-19 vaccine acceptance* – United States, May to July 2021. *COVID-19 vaccine acceptance was defined as those who had received the vaccine prior to or during pregnancy or planned to receive the vaccine during pregnancy; COVID-19 vaccine delay was defined as those who planned to receive COVID-19 vaccine, but after pregnancy; COVID-19 vaccine hesitancy was defined as those who were uncertain whether they would receive a COVID-19 vaccine; COVID-19 vaccine refusal was defined as those who had no plans to receive COVID-19 vaccine.
Pregnant persons who were recommended COVID-19 vaccination by their healthcare provider less commonly reported concerns about vaccine safety (35.5 % vs 55.9 %, P < 0.001), concerns about the vaccine not being tested among pregnant and breastfeeding individuals (48.8 % vs 36.9 %, P < 0.001), and concerns about vaccines being approved so quickly (26.8 % vs 49.3 %, P < 0.001) compared to respondents with no provider recommendation. Respondents whose provider recommended COVID-19 vaccination also more frequently reported believing COVID-19 vaccines are efficacious (77.1 % vs 45.4 %, P < 0.001) and that the benefits of COVID-19 vaccination outweigh the risks (86.4 % vs 51.7 %, P < 0.001) compared to respondents with no provider recommendation.
3.3. Factors associated with COVID-19 vaccine acceptance
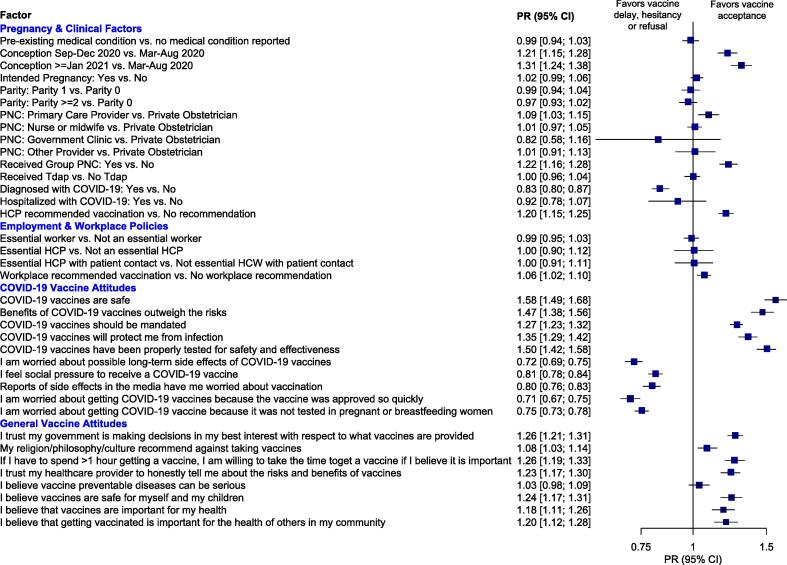
Attitudes toward COVID-19 vaccines and other vaccines were most strongly associated with COVID-19 vaccine acceptance during pregnancy. Vaccine acceptance was 2–3 times more likely among respondents who reported believing COVID-19 vaccines are safe (aPR 2.86; 95 % CI 2.49, 3.29), and COVID-19 vaccines have been tested properly for safety and effectiveness (aPR 2.54; 95 % CI 2.25, 2.86). Vaccine acceptance was significantly less likely among those who reported being worried that COVID-19 vaccines were approved so quickly (aPR 0.45; 95 % CI 0.40, 0.51), worried about possible long-term side effects of COVID-19 vaccines (aPR 0.47; 95 % CI 0.42, 0.52), and feeling social pressure to get vaccinated (aPR 0.62; 95 % CI 0.57, 0.68). In terms of attitudes toward other vaccines, trust in government to make vaccine decisions (aPR 1.71; 95 % CI 1.55, 1.87), belief that vaccines are safe for themselves and their children (aPR 1.53; 95 % CI 1.63; 95 % CI 1.42, 1.86), and trust that their healthcare provider was honest about risks and benefits of vaccines (aPR 1.61; 95 % CI 1.43, 1.82) were positively associated with COVID-19 vaccine acceptance (Fig. 3). Only belief that vaccine preventable diseases could be serious was not associated with COVID-19 vaccine acceptance during pregnancy (aPR 1.08; 95 % CI 0.96, 1.23).
Fig. 3.
Factors associated with COVID-19 vaccine acceptance* among US pregnant persons – May to July 2021. Abbreviations: CI, confidence interval; HCW, healthcare worker; OR, odds ratio; PNC, prenatal care. *Vaccine acceptance was defined as having received the vaccine prior to or during pregnancy or planning to receive the vaccine during pregnancy. NOTE: Prevalence ratios are adjusted for maternal age, race, region, birth overseas, urban/rural, educational attainment, employment status, insurance status.
Certain clinical and employment policies were also independently associated with vaccine acceptance. For example, although employment as an essential healthcare professional was not associated with vaccine acceptance during pregnancy after adjustment for other factors (aPR 1.01; 95 % CI 0.79, 1.29), having an employment policy recommending vaccination was positively associated with vaccine acceptance during pregnancy (aPR 1.15; 95 % CI 1.06, 1.26). Those participating in group prenatal care were 57 % more likely to accept COVID-19 vaccines compare to those not receiving group care (aPR 1.57; 95 % CI 1.40, 1.75), and those receiving prenatal care from a primary care provider were 22 % more likely to accept COVID-19 vaccines compared to those receiving prenatal care from a private obstetrician (aPR 1.22; 95 % CI 1.07, 1.39) (Fig. 3). Respondents who reported that their healthcare provider recommended COVID-19 vaccination were 52 % more likely to accept COVID-19 vaccines (aPR 1.52; 95 % CI 1.31, 1.76). Those who reported previous infection with COVID-19 (clinically or self-diagnosed) were less likely to accept COVID-19 vaccines during pregnancy (aPR 0.66; 95 % CI 0.59, 0.73). Pre-existing medical conditions were not significantly associated with COVID-19 vaccine acceptance during pregnancy (aPR 0.97; 95 % CI 0.88, 1.08).
4. Discussion
In this national survey of pregnant and recently pregnant US persons, we found that between December 2020 and July 2021, 8 % of respondents received ≥ 1 dose prior to pregnancy, 47 % received or planned to receive ≥ 1 dose during pregnancy, and another 27 % planned to receive COVID-19 vaccine after their pregnancy. While this estimate indicates as much as 55 % of pregnant persons could be protected from SARS-CoV-2 infection via vaccination, nearly-one-half of pregnant persons would remain vulnerable through the delay or refusal of COVID-19 vaccination during pregnancy. Concerns about the safety and benefits of COVID-19 vaccines were associated with vaccine delay and refusal, and these concerns were less common among those who received a COVID-19 vaccine recommendation by a healthcare provider. These issues should be addressed in future campaigns and interventions that aim to increase COVID-19 vaccination among pregnant persons.
Coverage of COVID-19 vaccination, in our sample and others, is lower among pregnant persons compared to the US general population. Data from Vaccine Safety Datalink for July 2021 indicate that as of July 2021, coverage of COVID-19 vaccine was 45 % among pregnant persons; 22 % had received COVID-19 vaccine before pregnancy, 19 % during pregnancy, and 4 % before and during pregnancy (CDC, 2022). In contrast, a national poll by Kaiser Family Foundation between July 15-27th found that 67 % of US adults had received at least one dose of COVID-19 vaccine and another 3 % planned to receive the vaccine (Kaiser Family Foundation, 2021). Results from US surveys have consistently shown that pregnant and breastfeeding individuals are less likely to accept COVID-19 vaccines compared to nonpregnant respondents (Sutton et al., 2021). While 76 % of nonpregnant respondents report acceptance of COVID-19 vaccines, 41 to 44 % of pregnant and 55 % of breastfeeding individuals were willing to accept COVID-19 vaccine (Sutton et al., 2021, Battarbee et al., 2022). National survey data have shown that COVID-19 vaccine coverage is 14–20 % lower among pregnant persons and those breastfeeding compared to non-pregnant women who are not attempting to conceive (Razzaghi et al., 2022). This problem is not isolated to the US. A global survey of vaccine acceptance among pregnant persons across 16 countries indicated that given a 90 % vaccine efficacy, 52 % of pregnant persons and 73 % of nonpregnant persons would receive a COVID-19 vaccine (Skjefte et al., 2021).
This low coverage and uptake stands in contrast to recommendations made by ACOG and Society for Maternal-Fetal Medicine (ACOG, 2020) and the continually expanding global recommendations for COVID-19 immunization during pregnancy (John Hopkins University, 2021). As a result, intervention is needed to improve immunization rates among pregnant individuals, both in the US and other countries with similar recommendations. We found the strongest factors associated with COVID-19 vaccine acceptance were linked with vaccine attitudes. Concerns about the safety of COVID-19 vaccines and the limited data specific to pregnant and breastfeeding individuals were commonly cited among those delaying or refusing COVID-19 vaccines. These findings align with those from a recent global survey, showing the strongest predictors of vaccine intentions identified globally was confidence in the vaccine safety and perceived effectiveness of COVID-19 vaccines (Skjefte et al., 2021). Interventions which target perceptions of and attitudes toward COVID-19 vaccines are needed to improve COVID-19 vaccine acceptance during pregnancy. These attitudinal barriers to vaccination are not unique to COVID-19 vaccines. Previous investigations of other recommended vaccines, including influenza and Tdap vaccines, have shown that safety concerns are a major reason for refusal of other recommended vaccines (Wilson et al., 2015, Myers, 2016). We found that while refusal of other recommended vaccines was common among those refusing COVID-19 vaccines, surprisingly, pregnant persons who delay COVID-19 vaccination frequently reported accepting other recommended vaccines. These results align with those from a recent systematic review, indicating that uptake of other recommended vaccines is higher compared to intention to receive COVID-19 vaccines (Shamshirsaz et al., 2022).
Importantly, we found that respondents who had received a COVID-19 vaccine recommendation less commonly reported safety concerns, more commonly believed in the health benefits of vaccination, and more frequently accepted COVID-19 vaccines. Given healthcare provider recommendation has been consistently identified in the literature as an important predictor of other vaccines recommended during pregnancy (Wilson et al., 2015, Myers, 2016, Mak et al., 2015), it is likely that conversations with healthcare providers may also help improve COVID-19 vaccination rates. Interventions at the provider level should be used to better support patient-provider conversations about COVID-19 vaccination. However, since receipt of a recommendation alone was modestly associated with higher COVID-19 vaccine acceptance, increasing the proportion of pregnant persons who receive a recommendation is likely insufficient on its own. Multilevel interventions targeting the patient, provider, and institutional levels are likely needed. Prior research for pertussis and influenza vaccines have shown that patient and provider education, increasing access to vaccines through on-site vaccination services, and implementing standing orders can be effective in increasing acceptance of recommended vaccines during pregnancy (Patel et al., 2021, Omer et al., 2022). Uptake of COVID-19 vaccines has increased since the implementation of this study. With 70 % of the US population entering pregnancy and <2 % receiving a vaccine during pregnancy, (CDC, 2022) similar strategies will likely be needed to encourage vaccination among the remaining 28 % of unvaccinated pregnancies.
This study had several strengths and weaknesses. First, we collected data from a large, diverse national sample of pregnancies six months following the introduction of COVID-19 vaccines in the US. We were able to capture a range of information related to health, pregnancy and beliefs and attitudes, which allowed us to comprehensively assess predictors of COVID-19 vaccine acceptance in this population. Despite this, we relied on self-reported immunization status. While previous research has shown that self-report is a reliable estimate of influenza vaccine status (Mak et al., 2015, King et al., 2018), the reliability of self-reported COVID-19 vaccine status has not yet been established. This may have resulted in an over or under-estimation of vaccine acceptance. Furthermore, although our sample included pregnancies from every US state, and we made additional efforts to ensure generalizability of our results, due to the nature of survey recruitment, our sample was restricted to those with internet access and may represent a selective subset of pregnant persons. We therefore cannot completely exclude the possibility of selection bias in our sample. This could limit the generalizability of our results. Finally, while this survey was useful for assessing COVID-19 vaccine attitudes during the six months following the introduction of COVID-19 vaccines in the US, acceptance of COVID-19 vaccines is likely to have improved since data collection, as demonstrate by the 70 % vaccine coverage currently observed among pregnant persons (CDC, 2022). Furthermore, because of the dynamic nature of the COVID-19 pandemic, including the emergence of more virulent variants of SARS-CoV-2 viruses, it is possible that vaccine acceptance among pregnant persons has changed. As a result, ongoing surveillance of COVID-19 vaccine behaviors and attitudes among pregnant persons is warranted.
5. Conclusion
Pregnant persons are a priority group for COVID-19 immunization. With 12 % of pregnant persons delaying vaccination until after pregnancy and 18 % indicating no plans to vaccinate, at least one-third of pregnant persons remained at higher risk for severe COVID-19. As with other recommended vaccines, concerns about vaccine safety appear to weigh heavily in the decision to delay or refuse COVID-19 vaccination. Interventions targeting patients and their providers which improve confidence in prenatal vaccination could be used to increase the proportion of pregnancies protected through vaccination.
Funding
This study was financially supported by a EuroQol fast-track award (#260-2020RA; PI Regan) and University of San Francisco Faculty Development Funds (PI Regan). The funder had no role in the conduct of the research, the interpretation of results or the decision to publish.
CRediT authorship contribution statement
Annette K. Regan: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Supervision, Project administration, Funding acquisition. Ravneet Kaur: Methodology, Validation, Formal analysis, Writing – review & editing. Marcianna Nosek: Methodology, Writing – review & editing, Funding acquisition. Pallavi A. Swathi: Investigation, Data curation, Writing – review & editing. Ning Yan Gu: Methodology, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study received financial support from the EuroQol Research Foundation (Grant #260-2020RA) and Faculty Development Funds from the University of San Francisco. The views expressed by the authors do not necessarily reflect the views of the EuroQol Research Foundation. The authors wish to additionally acknowledge the contributions made by the pregnant and postpartum participants across the US and US territories who contributed information to the present study.
Footnotes
Paper presentation information: Preliminary results from this research were presented at the 2021 ID Week, San Diego CA, September 29-October 3, 2021.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2022.101977.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data are not publicly available; however, data can be made available to other researchers upon reasonable request.
References
- ACOG. Practice Advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. December 2020 [cited 2021 March 1]; Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19.
- Adhikari E.H., SoRelle J.A., McIntire D.D., Spong C.Y. Increasing severity of COVID-19 in pregnancy with Delta (B.1.617.2) variant surge. Am J Obstet Gynecol. 2022;226(1):149–151. doi: 10.1016/j.ajog.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. m3320–m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battarbee A.N., Stockwell M.S., Varner M., Newes-Adeyi G., Daugherty M., Gyamfi-Bannerman C., Tita A.T., Vorwaller K., Vargas C., Subramaniam A., Reichle L., Galang R.R., Powers E., Lucca-Susana M., Parks M., Chen T.J., Razzaghi H., Dawood F.S. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol. 2022;39(01):075–083. doi: 10.1055/s-0041-1735878. [DOI] [PubMed] [Google Scholar]
- Blitz M.J., Gerber R.P., Gulersen M., Shan W., Rausch A.C., Prasannan L., Meirowitz N., Rochelson B. Preterm birth among women with and without severe acute respiratory syndrome coronavirus 2 infection. Acta Obstet Gynecol Scand. 2021;100(12):2253–2259. doi: 10.1111/aogs.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Pregnancy or breastfeeding. 2021 [cited 2021 September 27]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
- CDC. COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,* United States. 2022 [cited 2022 August 31]; Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women.
- DeSisto C.L., Wallace B., Simeone R.M., Polen K., Ko J.Y., Meaney-Delman D., Ellington S.R. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(47):1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley B.J.F., Mulder I.A., Di Mascio D., Vintzileos W.S., Vintzileos A.M., Berghella V., et al. Adverse pregnancy outcomes among individuals with and without severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2): a systematic review and meta-analysis. Obstet Gynecol. 2021;137(4):585–596. doi: 10.1097/AOG.0000000000004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D.D., Franco S.J. 2013 NCHS urban-rural classification scheme for counties. Vital Health Statistics. 2014;2(166):1–73. [PubMed] [Google Scholar]
- John Hopkins University. COVID-19 vaccine policies on pregnancy. 2021 [cited 2021 November 21]; Available from: https://www.comitglobal.org/explore/public-health-authorities/pregnancy.
- Kaiser Family Foundation. KFF COVID-19 Vaccine Monitor: July 2021. 2021 [cited 2021 October 1]; Available from: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-july-2021/.
- Kasehagen L., Byers P., Taylor K., Kittle T., Roberts C., Collier C., et al. COVID-19-associated deaths after SARS-CoV-2 infection during pregnancy - Mississippi, March 1, 2020-October 6, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(47):1646–1648. doi: 10.15585/mmwr.mm7047e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.P., McLean H.Q., Belongia E.A. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respir Viruses. 2018;12(6):808–813. doi: 10.1111/irv.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.Y., DeSisto C.L., Simeone R.M., Ellington S., Galang R.R., Oduyebo T., Gilboa S.M., Lavery A.M., Gundlapalli A.V., Shapiro-Mendoza C.K. Adverse pregnancy outcomes, maternal complications, and severe illness among US delivery hospitalizations with and without a Coronavirus disease 2019 (COVID-19) diagnosis. Clin Infect Dis. 2021;73(Supplement 1):S24–S31. doi: 10.1093/cid/ciab344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.B., Regan A.K., Joyce S., Gibbs R., Effler P.V. Antenatal care provider's advice is the key determinant of influenza vaccination uptake in pregnant women. Aust N Z J Obstet Gynaecol. 2015;55(2):131–137. doi: 10.1111/ajo.12292. [DOI] [PubMed] [Google Scholar]
- Myers K.L. Predictors of maternal vaccination in the United States: An integrative review of the literature. Vaccine. 2016;34(34):3942–3949. doi: 10.1016/j.vaccine.2016.06.042. [DOI] [PubMed] [Google Scholar]
- Oltean I., Tran J., Lawrence S., Ruschkowski B.A., Zeng N.a., Bardwell C., Nasr Y., de Nanassy J., El Demellawy D. Impact of SARS-CoV-2 on the clinical outcomes and placental pathology of pregnant women and their infants: a systematic review. Heliyon. 2021;7(3):e06393. doi: 10.1016/j.heliyon.2021.e06393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer S.B., O'Leary S.T., Bednarczyk R.A., Ellingson M.K., Spina C.I., Dudley M.Z., Chamberlain A.T., Limaye R.J., Brewer S.E., Frew P.M., Malik F.A., Orenstein W., Halsey N., Ault K., Salmon D.A. Multi-tiered intervention to increase maternal immunization coverage: a randomized, controlled trial. Vaccine. 2022;40(34):4955–4963. doi: 10.1016/j.vaccine.2022.06.055. [DOI] [PubMed] [Google Scholar]
- Patel K.M., Vazquez Guillamet L., Pischel L., Ellingson M.K., Bardají A., Omer S.B. Strategies to increase uptake of maternal pertussis vaccination. Expert Rev Vaccines. 2021;20(7):779–796. doi: 10.1080/14760584.2021.1940146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi H., Meghani M., Pingali C., Crane B., Naleway A., Weintraub E., Kenigsberg T.A., Lamias M.J., Irving S.A., Kauffman T.L., Vesco K.K., Daley M.F., DeSilva M., Donahue J., Getahun D., Glenn S., Hambidge S.J., Jackson L., Lipkind H.S., Nelson J., Zerbo O., Oduyebo T., Singleton J.A., Patel S.A. COVID-19 Vaccination coverage among pregnant women during pregnancy - eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(24):895–899. doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi H., Yankey D., Vashist K., Lu P.-J., Kriss J.L., Nguyen K.H., Lee J., Ellington S., Polen K., Bonner K., Jatlaoui T.C., Wilhelm E., Meaney-Delman D., Singleton J.A. COVID-19 vaccination coverage and intent among women aged 18–49 years by pregnancy status, United States, April-November 2021. Vaccine. 2022;40(32):4554–4563. doi: 10.1016/j.vaccine.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamshirsaz A.A., Hessami K., Morain S., Afshar Y., Nassr A.A., Arian S.E., Asl N.M., Aagaard K. Intention to receive COVID-19 vaccine during pregnancy: a systematic review and meta-analysis. Am J Perinatol. 2022;39(05):492–500. doi: 10.1055/a-1674-6120. [DOI] [PubMed] [Google Scholar]
- Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., Wu J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D., D'Alton M., Zhang Y., Kahe K., Cepin A., Goffman D., Staniczenko A., Yates H., Burgansky A., Coletta J., Williams Z., Gyamfi-Bannerman C. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obstet Gynecol MFM. 2021;3(5):100403. doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden N., Bunch K., Morris E., Simpson N., Gale C., O’Brien P., Quigley M., Brocklehurst P., Kurinczuk J.J., Knight M., Farrar D. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16(5):e0251123. doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.J., Paterson P., Jarrett C., Larson H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015;33(47):6420–6429. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., Azziz-Baumgartner E., Gilboa S.M., Meaney-Delman D., Akosa A., Bennett C., Burkel V., Chang D., Delaney A., Fox C., Griffin I., Hsia J., Krause K., Lewis E., Manning S., Mohamoud Y., Newton S., Neelam V., Olsen E.O., Perez M., Reynolds M., Riser A., Rivera M., Roth N.M., Sancken C., Shinde N., Smoots A., Snead M., Wallace B., Whitehill F., Whitehouse E., Zapata L. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available; however, data can be made available to other researchers upon reasonable request.