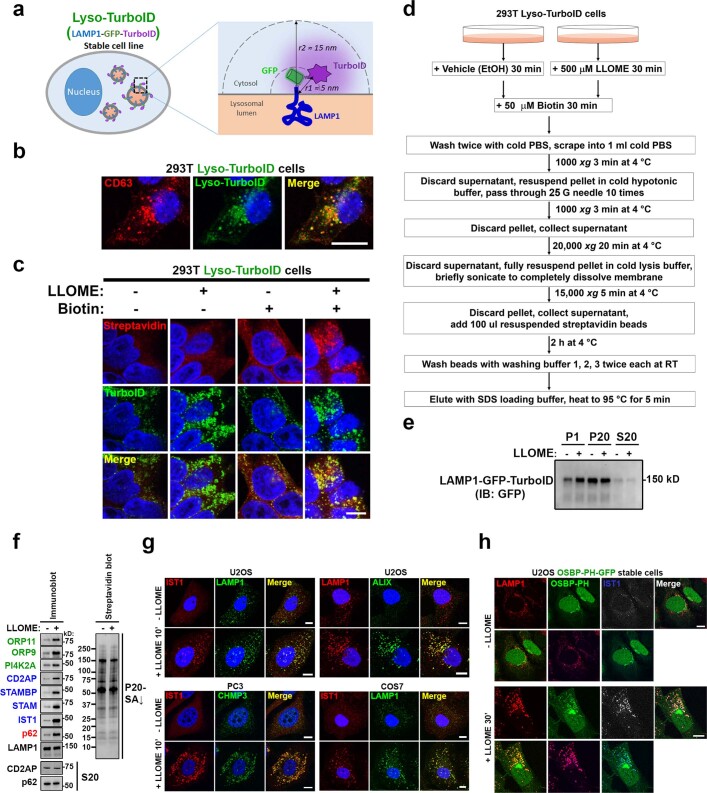
Extended Data Fig. 1. Identification of proteins enriched on damaged lysosomes using Lyso-TurboID cells.
a, Schematic illustration of Lyso-TurboID cells. r1 is the estimated distance between TurboID and the lysosomal membrane; r2 is the estimated radius within which a protein can be biotinylated by TurboID. b, Lyso-TurboID stably expressed in 293T cells colocalizes with the late endosome/lysosome marker CD63. c, Biotin addition enhances biotinylation of proteins that colocalize with Lyso-TurboID. Biotinylated proteins were stained by streptavidin. Note, lysosomal membrane damage by LLOME is known to cause lysosomal enlargement. 293T Lyso-TurboID (green) cells were treated with (500 μM) LLOME for 30 min, followed by another 30 min of biotin treatment (50 μM) without removal of LLOME. Cells were then fixed and permeabilized for streptavidin staining (red). d, Schematic illustration for the purification of proteins recruited to normal and damaged lysosomes. e, Lyso-TurboID is enriched in P20 (pellet after 20,000xg centrifugation) membrane fraction. Samples collected in (d) was analyzed by anti-GFP immunoblotting. Note that some lysosomes shifted from P20 to the heavier P1 fraction in LLOME-treated cells. This is likely because damaged lysosomes are extensively tethered to the ER (See Fig. 2) and thus a fraction of lysosomes might pellet with larger ER fragments in P1. f, Immunoblot analysis confirming the enrichment of mass spectrometry-identified proteins on damaged lysosomes. Protein samples were purified as in (a) from 293T Lyso-TurboID cells. S20, the supernatant above P20, contains the whole cytosol and some light membranes. g, The endosomal sorting complex required for transport (ESCRT) subunits IST1, ALIX, and CHMP3 are all rapidly recruited to damaged lysosomes in multiple cell lines. Cells were treated with 1 mM LLOME for 10 min and stained for the indicated endogenous ESCRT subunits and lysosomal marker LAMP1 after fixation and permeabilization. h, The PtdIns4P probe OSBP-PH-GFP is recruited to damaged lysosomes in U2OS cells as shown by the three channel colocalization between OSBP-PH, LAMP1, and IST1. Cells were treated with 1 mM LLOME for 30 min and then fixed for the staining of endogenous LAMP1 and IST1. DAPI stains the nuclei. Bar, 10 μm. Uncropped western blot images are provided in Supplementary Fig. 1.