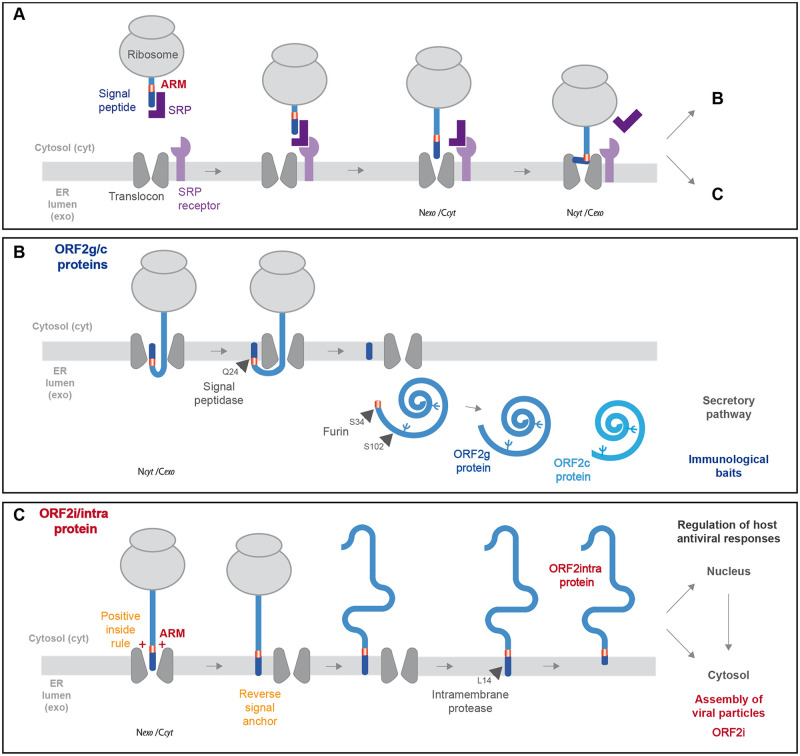
Fig 9. Model of ORF2 addressing regulation by ARM.
(A) The signal recognition particle (SRP) recognizes the hydrophobic signal peptide (SP) of the ORF2 nascent chain as it emerges from a translating ribosome. The ribosome-nascent chain-SRP complex is targeted to the membrane and interacts with the SRP receptor, resulting in the release of the SP and docking of the ribosome–nascent chain complex to the Sec61 translocon. The ORF2 SP initially inserts head-on in an Nexo/Ccyt orientation, then inverts its orientation to Ncyt/Cexo. (B) The C-terminal end of SP is exposed to ER lumen and is cleaved by signal peptidase, generating a new N-terminus. Translation then resumes, and the nascent ORF2 protein is translocated into the ER lumen where it is glycosylated and likely undergoes maturation by the proprotein convertase furin. This pathway generates the ORF2g/c forms. (C) For a fraction of ORF2 nascent polypeptide chains, the ARM leads the ORF2 SP to retain its Nexo/Ccyt orientation and integrates as reverse signal-anchor, according to the positive-inside rule. The ORF2 protein anchored to the cytosolic side of membrane is likely processed by an intramembrane protease to generate the ORF2i/ORF2 intra protein that is translocated into the nucleus and assembles into viral particles.