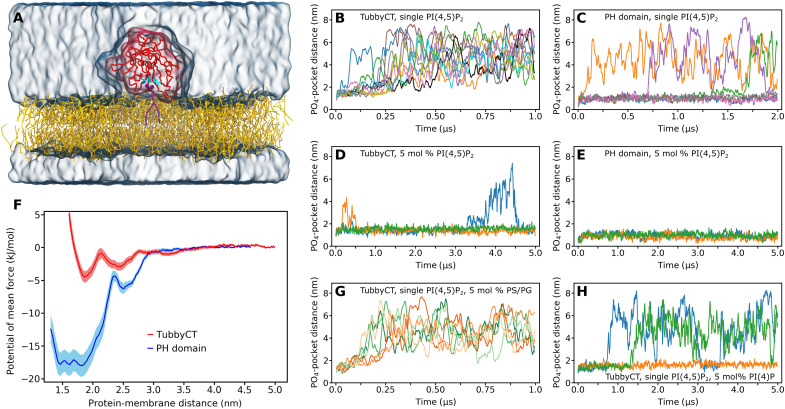
Fig. 1. Binding a single PI(4,5)P2 lipid does not target tubbyCT stably to a model membrane.
(A) CG system setup of tubbyCT (red) with one PI(4,5)P2 lipid (violet) in the binding pocket known from the crystal structure (cyan residues). The PI(4,5)P2 is embedded in a POPC bilayer (yellow); water and ions are shown as transparent surface. (B and C) Distance between the tubbyCT/PLCδ1-PH domain binding pocket and the phosphate layer (PO4 beads) of the binding leaflet containing a single PI(4,5)P2. Ten unbiased simulations of 1 μs [tubbyCT (B)] and seven unbiased simulations of 2 μs [PLCδ1-PH (C)] are shown, respectively. (D and E) Distance between the tubbyCT/PLCδ1-PH domain binding pocket and the phosphate layer (PO4 beads) of the binding leaflet containing 5 mol % of PI(4,5)P2. Three unbiased simulations of 5 μs each are shown. (F) Potential of mean force (PMF) for the PI(4,5)P2 binding of tubbyCT (red) and PLCδ1-PH domain (blue). (G) Control simulations of tubbyCT bound to one PI(4,5)P2 lipid embedded in a POPC membrane containing 5 mol % of POPS (PS) (green; 3 × 1 μs) and POPG (PG) (orange; 3 × 1 μs) lipids, respectively. (H) Control simulations of tubbyCT bound to one PI(4,5)P2 lipid embedded in a POPC membrane containing 5 mol % of PI(4)P (3 × 5 μs).