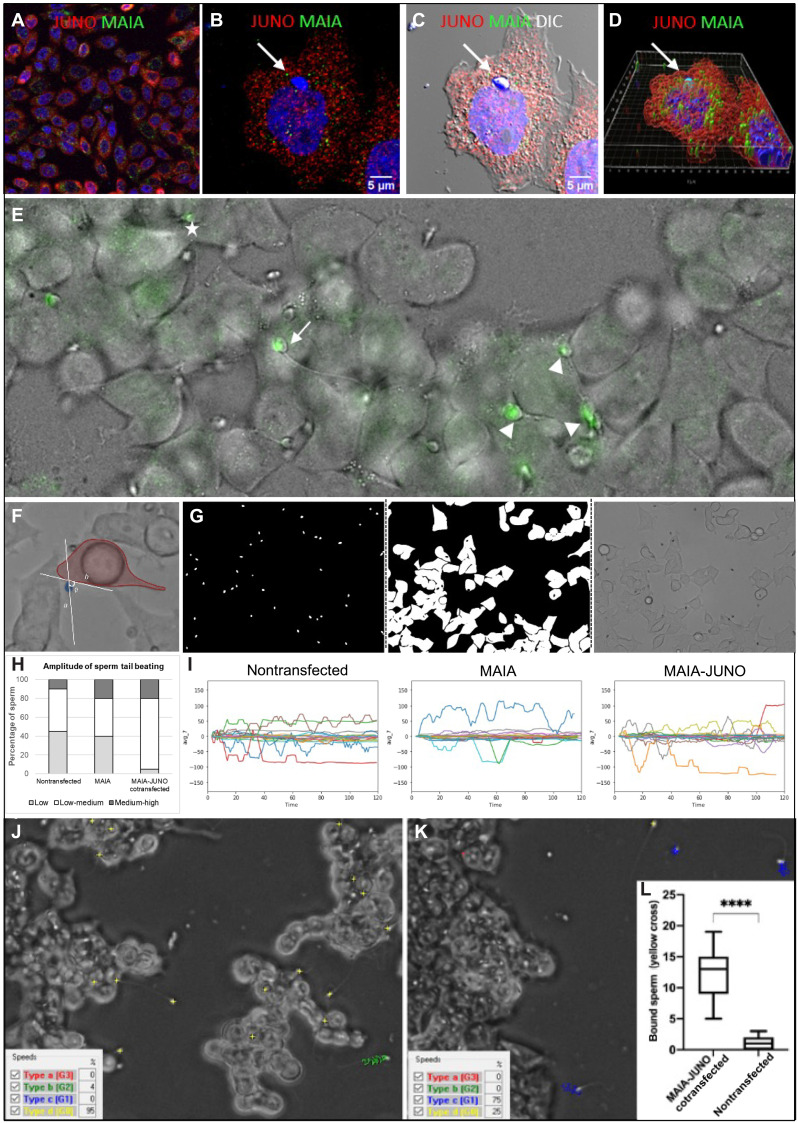
Fig. 3. Sperm binding assessment in relation to cell fusion.
(A) Immunostaining of MAIA (green) and JUNO (red) cotransfected CHO cells; (B) with fused human sperm head (white arrow); nuclear counterstain (Hoechst); (C) merged with DIC; (D) Imaris Surface Render analysis (fig. S4 and movie S4). (E) Representative image of sperm captured by lifetime confocal microscopy (green) displaying a range of tail beating reflecting the status of sperm-cell fusion (movie S5); white arrow, fusing sperm (slow tail beating); white asterisks, nonfusing sperm (fast tail beating); white tips (fused sperm, static tail without beating). (F to I) AI object identification. (F) Representative image of captured tail beating pattern used for AI training. (G) Example of novel neuronal network training masks for human sperm tail beating pattern quantification. (H) Quantification of sperm tail beating amplitude calculated from the degree angle as low, medium, and high. (I) Representative charts of degree angle change. (J and K) Representative snapshot of a video recording of sperm kinematic parameter analysis assessed by CASA with phase contrast (yellow cross, static sperm–bound sperm with low tail beating; blue track, medium-progressive swimming sperm; green track, rapid-swimming sperm; red track, rapid-progressive swimming sperm); (J) MAIA-JUNO cotransfected HEK293T (HEK) cells (movie S6); (K) nontransfected HEK cells (movie S7). (L) Quantification of video recordings (ncotransfected = 17 and nnontransfected = 14) of bound sperm (yellow cross) assessed by CASA, ****P ≤ 0.0001.