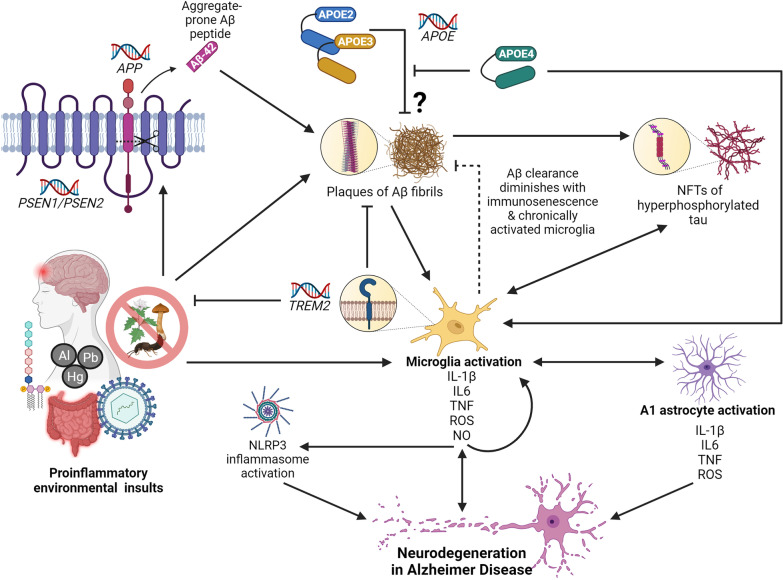
Fig. 2.
The role of neuroinflammation in Alzheimer disease (AD). Microglia activation, and the subsequent release of pro-inflammatory cytokines, ROS, and NO, is a central event in the onset of neurodegeneration in AD. Pro-inflammatory microglia release cytokines that cause a feed-forward loop of microglia activation, activate the NLRP3 inflammasome, and facilitate cross-talk with A1 astrocytes, all of which coalesce on neurodegenerative pathways. In AD, microglia can be activated upon detection of PAMPs, DAMPs, and other pro-inflammatory molecules as a result of environmental insult—including gut dysbiosis and sustained exposure to LPS endotoxin, viral infection, and TBI. These pro-inflammatory environmental insults are also associated with accelerated amyloid β (Aβ) plaque deposition and the overexpression of APP and β-secretase (PSEN1/PSEN2). Mutations in PSEN1 and PSEN2 can result in the cleavage of APP to produce 42-residue, aggregate-prone peptides, and mutations in APP can lead to overproduction, misfolding, and formation of Aβ fibrils. Microglia become activated upon sensing DAMPs from Aβ plaques, and are sequestered to clear them. However, chronically activated microglia and immunosenescence diminish the efficacy of this ability over time. TREM2 modulates microglia activity and survival. Mutations in TREM2 can impact the ability of microglia to modulate cytokine production, phagocytose bacteria, and clear neural debris. When Aβ plaques are not cleared, they, and the chronically activated microglia, can induce the formation of NFTs. Hyperphosphorylated tau protein can further activate microglia. Although their exact mechanism of action is unknown, the APOE2 and APOE3 isoforms are protective against AD and are thought to play a role in clearing Aβ plaques. The APOE4 isoform, however, is unable to clear Aβ plaques and forms neurotoxic fragments that can activate microglia