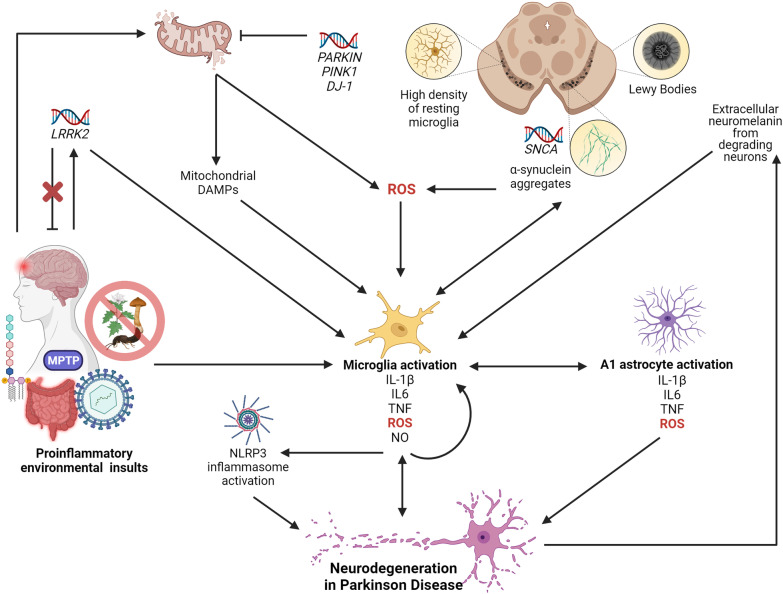
Fig. 3.
The role of neuroinflammation in Parkinson disease (PD). In PD, the SN is preferentially vulnerable to neuron loss. The high density of resting microglia in this region (12% of cells—the highest in the brain) is thought to render the SN vulnerable to the effects of a robust inflammatory response. Furthermore, neurodegeneration in the SN can cause a feed-forward loop, where, upon neuronal degradation, the high concentration of neuromelanin in the SN is released into the extracellular space and is cleared by activated microglia. Microglia activation, and the subsequent release of pro-inflammatory cytokines, ROS, and NO, is also a central event in the onset of neurodegeneration in PD. Like in AD, pro-inflammatory microglia release cytokines that cause a feed-forward loop of chronic microglia activation, activate the NLRP3 inflammasome, and facilitate cross-talk with A1 astrocytes, all of which coalesce on neurodegenerative pathways. In PD, microglia can be activated upon detection of PAMPs, DAMPs, and other pro-inflammatory molecules as a result of environmental insult—including gut dysbiosis and sustained exposure to LPS endotoxin, viral infection, TBI, MPTP, and pesticides. These pro-inflammatory insults can result in the overexpression of LRRK2, which is also pro-inflammatory and can modulate cytokine production. Mutations in LRRK2 can modulate PD risk, but gene knockouts impair microglia activation and are protective against pro-inflammatory environmental insults. Environmental insults further activate microglia through mitochondrial-mediated toxicity. Pesticides inhibit NADH dehydrogenase, which can lead to the production of ROS and mitochondrial DAMPs, both of which activate microglia. PARKIN, PINK1, and DJ-1 play roles in the clearance of damaged mitochondria, and mutations in these genes can prevent proper clearance and the production of ROS and DAMPs. Pathological hallmarks of PD are aggregates of SNCA surrounded by Lewy Bodies. SNCA mutations, amplification, and overexpression, can result in the formation of these protein aggregates, which are identified and cleared by DAMP-sensing microglia. SNCA can also produce ROS, which interacts with the P2X7 receptor on microglia