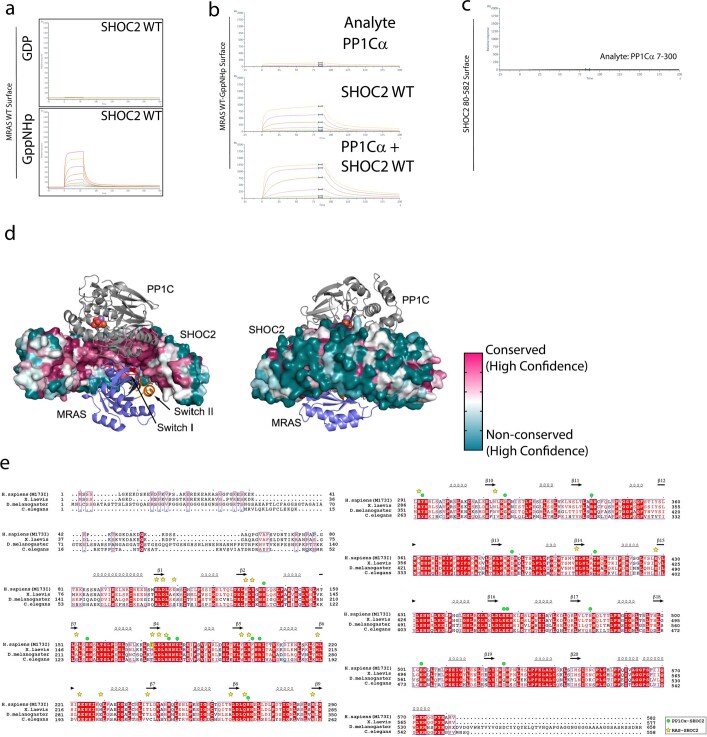
Extended Data Fig. 1. SHOC2 binds MRAS and PP1 through the highly conserved concave face.
a. Recombinant MRAS loaded with either GDP or non-hydrolysable GTP analogue GppNHp was immobilized on a streptavidin (SA) chip and recombinant SHOC2 80-582 was flowed over in dose response. Representative sensorgrams are shown, where only active GppNHp loaded MRAS could bind to SHOC2. b. Recombinant MRAS loaded with non-hydrolysable GTP analogue GppNHp was immobilized on a streptavidin (SA) chip and recombinant PP1Cα, SHOC2 80-582 or FL SHOC2 was flowed over in dose response in presence of excess PP1Cα. Representative sensorgrams are shown, where PP1Cαbinds to active GppNHp loaded MRAS only on the presence of SHOC2 80-582. c. Recombinant SHOC2 80-582 was immobilized on a streptavidin (SA) chip and recombinant PP1Cαwas flowed over in dose response. d. PP1C and MRAS bind a highly conserved surface on SHOC2. Front and back views of the ternary SHOC2/MRAS/PP1C complex. Note that SHOC2 engages PP1C and MRAS with a highly conserved region on its concave surface. By contrast, the outer, convex surface of SHOC2 is poorly conserved. The surface of SHOC2 is coloured according to conservation from magenta (most conserved) to teal (most variable) as analysed with the CONSURF server (PMID: 27166375). e. SHOC2 sequence alignment. Amino acid sequences of human, frog, fly and worm SHOC2 are aligned and identically conserved residues are shaded red. The site of the M173I mutation in the present structure is shaded yellow. Secondary structure elements of SHOC2 are indicated above the alignment. Symbols above the alignment indicate residues that in the holoenzyme complex lie in the interface with PP1Cα (green dots) or MRAS (yellow stars).