Abstract
The COVID-19 pandemic has challenged the continued delivery of healthcare globally. Due to disease risk, clinicians were forced to re-evaluate the safety and priorities of pre-pandemic care. Neuro-oncology presents unique challenges, as patients can deteriorate rapidly without intervention. These challenges were also observed in countries with reduced COVID-19 burden with centres required to rapidly develop strategies to maintain efficient and equitable care. This review aims to summarise the impact of the pandemic on clinical care and research within the practice of Neuro-oncology. A narrative review of the literature was performed using MEDLINE and EMBASS and results screened using PRISMA guidelines with relevant inclusion and exclusion criteria. Search strategies included variations of ‘Neuro-oncology’ combined with COVID-19 and other clinical-related terms. Most adult and paediatric neurosurgical centres experienced reductions in new referrals and operations for brain malignancies, and those who did present for treatment frequently had operations cancelled or delayed. Many radiation therapy and medical oncology centres altered treatment plans to mitigate COVID-19 risk for patients and staff. New protocols were developed that aimed to reduce in-person visits and reduce the risk of developing severe complications from COVID-19. The COVID-19 pandemic has presented many challenges to the provision of safe and accessible healthcare. Despite these challenges, some benefits to healthcare provision such as the use of telemedicine are likely to remain in future practice. Neuro-oncology staff must remain vigilant to ensure patient and staff safety.
Abbreviations: COVID-19, Coronavirus virus disease 2019; ICU, Intensive Care Unit; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; CNS, Central nervous system; NSA, Neurosurgical Society of Australasia; OECD, Organisation for Economic Co-operation and Development; USA, The United States of America; RT, Radiation therapy; ASCO, American Society of Clinical Oncology; HGG, High-grade glioma; LGG, Low-grade glioma; PPE, Personal protective equipment; TSS, Trans-sphenoidal surgery; UK, The United Kingdom; WHO, The World Health Organisation; PCR, Polymerase chain reaction; MGMT, O[6]-methylguanine-DNA methyltransferase; TTFs, Tumour-treating fields; PCV, Procarbazine, lomustine (CCNU), vincristine (chemotherapy regimen)
Keywords: Neuro-oncology, Neurosurgery, Surgery, Radiation therapy, Medical oncology, paediatric Neuro-oncology, Neuro-oncology research, Glioma, COVID-19, Coronavirus, SARS-CoV-2, Pandemic, Impact, Telemedicine
1. Introduction
Since its emergence in late 2019 [1], the COVID-19 pandemic has had a dramatic impact across all aspects of society. While hospitalisations and ICU admissions have continued to rise, clinicians have been forced to rationalise non-emergency care within already-strained healthcare systems. This dilemma is particularly relevant in Neuro-oncology, where patients with brain cancer can become critically unwell if treatment is delayed [2]. Caused by the SARS-CoV-2 virus, COVID-19 infection is known to have neurological sequelae, with hypothesised CNS infection mediated via angiotensin converting enzyme receptors present on endothelial, glial and neuronal cells [3]. It is hypothesized that patients with brain cancer are more susceptible to such manifestations due to an already injured brain [4]. In view of disease risk, Neuro-oncology clinics have had to develop new guidelines which balance treatment benefits with infection risk to patients and staff. Consequently, many Neuro-oncology centres reported treatment delays, modifications and cancellations, that was compounded by reduced funding, as COVID-19 care and research became the priority [5].
The impacts of the COVID-19 pandemic have varied globally. Areas with high mobility and trade had high case rates, whilst many island nations were able to remain largely virus-free with strict border closures [6]. Additionally, people living in highly-populated areas without clean water and sanitation systems had higher rates of infection. Further disparities exist in developing countries, where vaccine rates are lower than OECD countries [7]. The Australian COVID-19 pandemic experience has differed from the wider global response. As a geographically isolated island, Australia was able to combine strong border closures with non-pharmacological public health measures to “flatten the curve” in 2020 [8]. The COVID-19 response prioritised protection of vulnerable people, provision of treatment and support to affected people, maintenance of regular health services, protection of healthcare workers and provision of mental health services [9]. Consequently, Australia has largely avoided the burden of disease faced elsewhere, with 5.3 deaths per 100,000 people as of October 2021, compared to 212 deaths per 100,000 people in the USA [10]. Nationwide lockdowns and restrictions on in-person medical appointments have threatened the provision of regular healthcare. Increases in telemedicine utilisation has permitted treatment continuation while reducing physical interactions with health services [11]. Although such changes have been effective, they may inadvertently lead to health inequality. For example, cultural barriers preclude many of Australia’s First Nations peoples from engaging with telehealth [12], whilst public health messages have not always been optimally conveyed to non-English speakers, leading to varying levels of compliance [13]. Similarly, as of January 2022, Australia’s First Nations peoples have lower vaccination rates than wider Australia [14]. Reasons for this include vaccine hesitancy, complacency and poor public health messaging.
The impacts of COVID-19 on Neuro-oncology span many aspects of care. Neurosurgical management of CNS tumours is complex and reliant on high acuity inpatient care, increasing the risk of nosocomial COVID-19 infection and staffing strain. Neurosurgical operations also present risks to operating room staff, due to close proximity to the airway, aerosol-generating techniques and general anaesthetic requirements [15]. The NSA released a position statement outlining the maintenance of Neuro-oncological operations throughout the pandemic, particularly for acute emergencies and CNS tumours with the potential for quick deterioration [16]. In some centres, RT-only therapy was instituted whenever there was thought to be clinical equipoise, allowing patients to avoid surgery [17]. These perspectives are shared globally due to the health risks of postponement [18]. The COVID-19 pandemic has also impacted RT delivery for CNS tumours as regular visits to healthcare centres are necessary. To help mitigate this, guidelines were established to reduce patient interaction with healthcare workers, including opting for hypofractionated regimens [19]. Furthermore, medical management of Neuro-oncology patients remains a topic of contention during the COVID-19 pandemic. Lymphopaenic patients are at higher risk of viral complications, which forces clinicians to question the use of myelotoxic drugs such as temozolomide [19]. ASCO encouraged careful consideration of immunosuppressant requirement during the pandemic [20].
The aim of this review is to highlight the global impact of the COVID-19 pandemic on Neuro-oncological practice. This involves analysis of the centre-based changes as reported in the literature, as well as alterations to triage and treatment protocols. This paper explores the impacts on neurosurgery, radiation therapy, medical oncology, the paediatric cohort, and Neuro-oncological research. Finally, we outline future recommendations and key lessons learned during the pandemic.
2. Methods and results
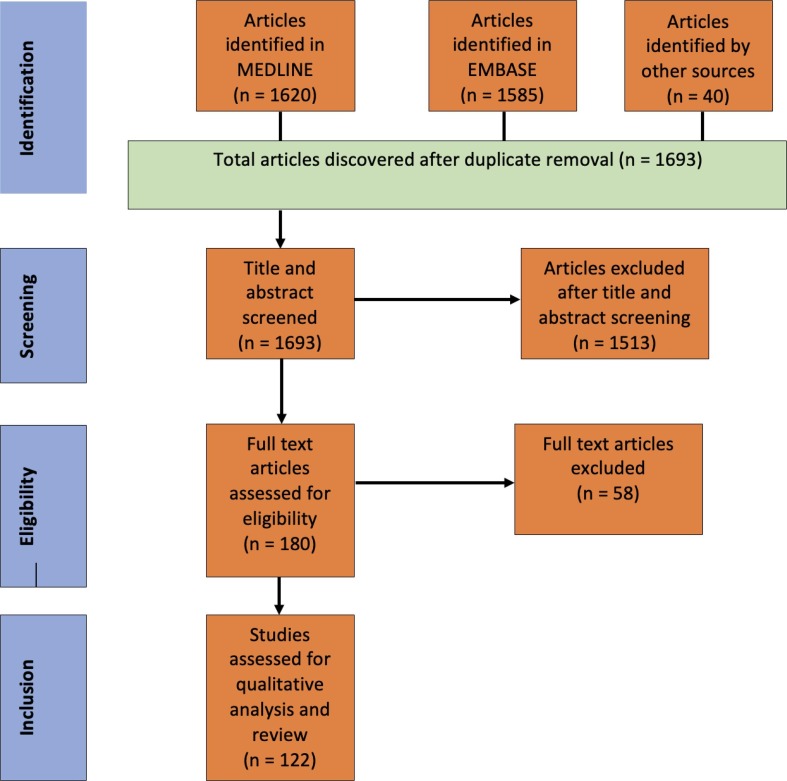
The methodology utilised is outlined in the PRISMA guidelines. Articles were searched for in the MEDLINE and EMBASE databases on September 20th, 2021. Search strategies used variations of ‘Neuro-oncology’ in combination with variations on ‘neurosurgery’, ‘surgery’, ‘anaesthetics’ ‘radiation therapy’, ‘medical oncology’, ‘paediatric’ and ‘research’. Results were then combined with the COVID-19 filter to produce 1653 unique articles. An additional forty papers were obtained from other articles’ reference lists and from experts in the field. Inclusion criteria included English, Spanish and French papers due to author proficiency, and COVID-19-articles were restricted to the period November 2019 – September 2021. After screening all titles and abstracts (performed by one person), 1513 articles were excluded as they were not relevant to Neuro-oncology. Further review of full text articles allowed for the exclusion of fifty-eight studies due to lack of relevance to clinical care and research. 122 articles remained, which have been referenced in this manuscript (Fig. 1 )
Fig. 1.
PRISMA flow diagram; EMBASE and MEDLINE were systematically searched for articles pertaining to the impact of the COVID-19 pandemic on Neuro-oncology. Of 1693 articles screened, 122 satisfied criteria for qualitative review.
All centres maintained neurosurgical treatment for CNS tumours to some degree, however, it is clear that the pandemic obstructed the regular delivery of care (Table 1 ). Many centres described a reduction in new referrals and Neuro-oncological procedures [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], although emergency operations largely remained unaffected. One centre described how a national COVID-19 vaccination campaign allowed them to recommence operations for brain tumours [37]. Another centre reported that glioblastoma cases were more aggressive than the previous year, with simple diagnostic biopsy often being management of choice [29]. Some centres described altered treatment pathways, for example, opting for either RT alone or in combination with surgery [27], or cancelling operations and offering purely supportive care when benefit from active treatment was considered limited [28]. Additionally, one study found that disruptions to care were greater in centres without a dedicated surgical oncology list [28]. Many centres continued outpatient appointments via telemedicine [22], [36], [39], [40].
Table 1.
Summary of clinical studies investigating the impact of the COVID-19 pandemic on neurosurgical management of Neuro-oncological patients.
| Author | Centre | Type of Study | Number in Study | Time period | Effect of pandemic |
|---|---|---|---|---|---|
| Khalafallah et al, 2020 [39] | Department of Neurosurgery, Johns Hopkins University School of Medicine, USA | Retrospective review | Mar 2020 vs Apr 2020 | Cancellation of tumour operations (%, 2nd highest). Reduced clinic visits (97 %), majority conducted on telemedicine (92.62 %) | |
| Kilgore et al, 2021 [21] | Ochsner Medical Centre and Tulane Medical Centre, USA | Retrospective review | Mar-Jun 2020 vs equivalent period in 2017–2019 | 17 % reduction in craniotomies for tumour cases | |
| Luther et al, 2021 [22] | Department of Neurosurgery, University of Miami Miller School of Medicine, USA | Retrospective review | Analysis over 23/3/20–20/7/20 period | Reduced surgical cases in the two pandemic peaks, telemedicine visits maintained post-quarantine | |
| Mallari et al, 2021 [115] | Department of Surgery, Ohio State University Wexner Medical Centre, USA | Retrospective review | Mar 2020-Jan 2021 vs Mar 2019-Jan 2020 | Reduced ICU utilisation, LOS (cranial cases) and ICU LOS. No changes in preop. ASA scores, resection/remission rates, readmissions, reoperations | |
| Noureldine et al, 2020 [55] | Department of Neurosurgery, University of South Florida Morsani College of Medicine, Tampa General Hospital, Tampa, USA | Retrospective review | Ninety-one (91) patients in 2020 period, two-hundred and fourteen (2 1 4) patients/4 weeks in 2019 | 23/3/20–20/4/20 vs 1/1/19–30/6/19 | Mild increase in number of craniotomies/biopsies for CNS tumours |
| Saad et al, 2020 [23] | Department of Neurosurgery, Emory University Hospital, USA | Retrospective review | Seven-hundred and fifty-one (7 5 1) patients across whole period | 16/3/20–15/4/20 vs equivalent periods 2017–2019 | Reduced case volume for tumour operations (55 %). No endonasal surgeries performed (average of 15 performed 2017–2019) |
| Wali et al, 2021 [24] | Department of Neurosurgery, University of San Diego, USA | Retrospective review | 24/11/19–6/7/20 (pre vs post pandemic split at 16/3/20) | Mild reduction in number of cranial tumour ops. (95 → 77). Mild reduction in endoscopic endonasal approach/TSS (14 → 9) | |
| Norman et al, 2021 [40] | Department of Neurological Surgery, Weill Cornell Medicine, New York, USA | Retrospective review | 278 patients | 13/3/20–1/5/20 vs 13/3/19–1/5/19 | Greater treatment delays in pandemic period though no changes In outcome, patients who utilised telemedicine had more stable tumour control than those who had office visits |
| Soriano Sanchez et al, 2020 [56] | Internet-based Survey, Latin American Federation of Neurosurgical Societies | Cross-sectional study | Four hundred and eighty-six (4 8 6) responses | CNS tumours remained third highest indication for emergency ops. Surgeons infected with COVID-19 in Ecuador, Paraguay, Costa Rica | |
| Ahuja et al, 2020 [25] | Welsh Centre for Spinal Surgery and Trauma, University Hospital of Wales, Heath Park, Cardiff, UK | Retrospective review | 1/2/20 –30/4/20 vs 1/2/19–30/4/19 | Reduced referrals for spinal tumours (∼33 %) | |
| Amoo et al, 2021 [116] | National Centre for Neurosurgery, Beaumont Hospital, Ireland | Retrospective review | Mar -May 2020 vs Mar-May 2019 | No reduction in mean times to hospital transfer or surgical admission No change in 30-day M&M |
|
| Ashkan et al, 2021 [26] | King’s College Hospital NHS Foundation Trust, UK | Prospective cohort study | 18/3/20–15/5/20 vs 18/1/20–17/3/20 | Reduced emergency referrals with Neuro-oncology cause (210 → 171) Reduced number of operations for HGG (31 → 12) and number of Neuro-oncology referrals (443 → 275) | |
| Fountain et al, 2021 [27] | Fifteen Neuro-oncology Centres in the UK | Prospective cohort study | One thousand three-hundred and fifty-seven (1357) referrals for newly diagnosed or recurrent intracranial tumours | Patients identified from 1/4/20–31/5/20 with thirty day follow up | 88 % of planned ops. performed. LGGs actively monitored. 9 % of newly diagnosed HGG patients received support/fractionated RT. Low rates of COVID-19 infection in neurosurgical patients |
| Price et al, 2020 [28] | Survey of eighteen neurosurgical units, UK | Prospective survey | One thousand two-hundred and twenty-one (1221) patients | Survey performed between 23/3/20–23/4/20 (two weeks pre/post COVID-19 infection peak) | Reduced number of new patients per week (27 %). 10.7 % of patients had a change in initial management (majority cancellation, mostly elderly, LGG) |
| Richardson et al, 2021 [38] | Sixteen UK and Republic of Ireland Neurosurgical Centres; Single-centre Analysis of The Walton Centre, Liverpool, England | Retrospective cross-sectional cohort study | April-June 2020 vs equivalent period 2019 | Reduction in number of glioblastoma cases (30 → 24) although represented a greater proportion of cases (3.6 % →8.3 %) | |
| Dannhoff et al, 2021 [29] | Strasbourg University Hospital, France | Retrospective analysis with prospectively gathered cohort | One hundred and sixty patients (1 6 0) received neurosurgical care | Patients gathered 15/3/20–12/5/20 vs 15/3/19–12/5/19 | Reduced number of Neuro-oncology operations (53 → 27) but mild increase in % composition within neurosurgery. Glioblastoma presentations noted more aggressive than 2019 |
| Doglietto et al, 2020 [117] | Hospitals in Eastern Lombardy, Italy | Prospective analysis | One hundred and twenty-three (1 2 3) patients over ten hospitals | Pre and post-op. questionnaires completed by patients after lockdown lifted | Higher anxiety in Neuro-oncology patients compared to non-oncology neurosurgery patients. No patients developed COVID-19 infection post-operatively |
| Vissio et al, 2021 [118] | Analysis of Surgical Oncological Pathology, University Hospital of Turin, Italy | Retrospective analysis | Twenty-three (23) CNS samples 2020, 15–24 CNS samples 2017–2019 | 9/3/20–8/5/20 vs equivalent periods 2017–2019 | Similar number of CNS samples, no changes in WHO grading of samples |
| Krenzlin et al, 2020 [119] | Neurosurgical Departments, University Medical Centre Mainz and University Medical Centre Göttingen, Germany | Retrospective cohort study | Two-hundred and forty-three (2 4 3) patients over all areas of neurosurgery | 16/3/20–16/4/20 vs equivalent periods 2018–2019 | Reduced admissions due to brain tumours (61.1 % +/- 38.8 %) |
| Zahrou et al, 2021 [37] | Department of Neurosurgery, Ibn Tofail Hospital, Mohammed VI University Hospital, Marrakesh, Morocco | Retrospective review | 2/3/21–28/6/21 (post-vaccination) vs 2/3/20–28/6/20 (pandemic) vs 2/3/19–28/6/19 (pre-pandemic) | Reduction in number of tumour operations in pandemic period compared to pre-pandemic (64 → 34), increased to 58 after introduction of COVID-19 vaccine | |
| Khosravi et al, 2020 [30] | Rasool-e-Akram Hospital, Iran | Retrospective review | 18/2/20–15/4/20 vs equivalent period 2019 | Reduced number of operations for brain tumours (36 → 19) and spinal cord tumours (2 → 0) | |
| Goyal et al, 2021 [32] | All India Institute of Medical Sciences, India | Partial-retrospective and partial-prospective analysis | One-hundred and sixty-four (1 6 4) patients over all areas of neurosurgery | 25/3/20–31/5/20, compared to 25/3/19–31/5/19 | Reduced number of Neuro-oncological operations [36 (30 cranial, 6 spinal) → 14 (12 cranial, 2 spinal)], |
| Singh et al, 2021 [120] | Institute of Medical Sciences, Banaras Hindu University, India | Retrospective and prospective study | 1/1/20–31/5/20 | Small increase in Neuro-oncological indication for emergency neurosurgery during pandemic (1.1 % →4%) | |
| Wang et al, 2021 [103] | Department of Neurosurgery, Zhongnan Hospital of Wuhan University, Wuhan, China | Letter to the editor | February-July 2020 | Dramatic increase in number of brain tumour operations from pre to post-closure period (∼20→∼180), increased recurrence of some malignant brain tumours resected pre-pandemic | |
| Hameed et al, 2021 [31] | One-hundred and forty-four Asian Hospital survey by Chinese Society of Neuro-oncology | Cross-sectional study | Three-thousand six hundred and ninety-nine (3699) neurosurgeons | 1/4/20–18/4/20 | Reduced number of oncology ops. (25–50 %). Centres resumed elective surgery in only COVID-19-neg patients (67.4 %), only performed emergency cases (11.1 %). suspended all neurosurgical activity (2.1 %), moved neurosurgical personnel to other departments (63.2 %) |
| Lee et al, 2021 [81] | Chung-Ang University College of Medicine, Seoul, South Korea | Retrospective review | 1/2/20–30/6/20 vs 1/2/19–30/6/19 | Increased ‘time interval to skin incision’ but no changes to outcomes. Reduced number of craniotomies (28 → 10) | |
| Manusubroto et al, 2020 [33] | Department of Neurosurgery, Dr Sardjuto General Hospital, Indonesia | Retrospective review | 2/2/20–10/4/20 vs preceding 9 weeks | Reduced number of Neuro-oncological emergency cases. Tumour resection highest indication for ops. Elective ops. maintained against national guidelines | |
| Suryaningtyas et al, 2020 [34] | Surabaya Academic Tertiary Hospital, Indonesia | Retrospective analysis | 1/1/20–14/6/20 vs Apr 2020-future | Reduced number of ops. (60 → 18) | |
| Antony et al, 2020 [35] | Five Neurosurgery Centres, Australia (four adult, one paediatric) | Prospective observational study | One-thousand two-hundred and ninety-eight (1298) admissions over all areas of neurosurgery | Feb-Apr 2020 | Reduced number of ops., no change in number of oncological emergencies. No patients tested positive to COVID-19 |
| Mrugala et al, 2021 [36] | International Survey of twenty-one Neuro-oncology organisations across 6 continents | Cross-sectional study | Five hundred and eighty-two (5 8 2) respondents (45 % US, 55 % non-US) | Surveys collected between 24/4/20–17/5/20 | Elective cases re-scheduled (60 %) or cancelled (37 %). 14.3 % of cases planned with endonasal approach were converted to craniotomy. 95 % of respondents converted aspects of practice to telemedicine |
Abbreviations: USA – The United States of America; UK – The United Kingdom; NHS – National Health Service; LOS – length of stay; ICU – Intensive Care Unit; ops. – operations; TSS – trans-sphenoidal surgery; M&M – morbidity and mortality; HGG – high-grade glioma; LGG – low-grade glioma; RT – radiotherapy; LOS – length of stay.
Most RT centres experienced changes in treatment plans, often opting for hypofractionated schedules (Table 2 ). This involves delivering the same dose over shorter time periods to reduce the risk of exposure. As a result, many centres had reduced number of visits [41]. Some centres also reported reduced patient numbers [41], [42], [43]. Additionally, clinicians held concerns regarding the use of immunosuppressive agents such as temozolomide [36].
Table 2.
Summary of clinical studies investigating the impact of the COVID-19 pandemic on radiation therapy and medical oncology management of Neuro-oncological patients.
| Author | Centre | Type of Study | Number in Study | Time Period | Effect of Pandemic |
|---|---|---|---|---|---|
| Pendyala et al, 2021 [98] | Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, Robert Wood Johnson Barnabas Health, New Jersey, USA | Retrospective review | Five-hundred and forty-five (5 4 5) patients total, sixteen (16) glioma | 9/3/20–15/6/20 | Two patients advised against RT due to COVID-19 risk). Two elderly patients received hypofractionated RT for glioblastoma |
| Roberge et al, [42] | Radiation Oncology Department, Centre Hospitalier de l’Université de Montréal (CHUM), Canada 2020 | Prospective review | 13/3/20–10/8/20 vs 13/3/19–10/8/19 | Reduced treatment of CNS tumours (-5% primary CNS, −21 % metastases) | |
| Martinez et al, 2020 [57] | Latin American Survey of Radiation Oncologists | Cross-sectional study | Responses from one to twenty-seven (1–27) radiation oncologists per centre from one-hundred and fifteen (1 1 5) RT services | 6/5/20–30/5/20 | 24.3 % reported delays in treatment of LGG |
| Samper Ots et al, 2021 [121] | Survey of Sixty-Six Radiation Oncology Departments, Spain | Retrospective review | Two-hundred and thirty-five (2 3 5) COVID-19 positive patients | 15/2/20–15/5/20 | Brain metastases were a risk factor for death from COVID-19 (50 % mortality, n = 22) |
| Spencer et al, 2021 [41] | Population study of all RT delivered, UK | Retrospective analysis | Feb-Jun 2020 vs equivalent months in 2019 | Reduced RT courses (10.6–28.4 %). Reduced in-patient visits (20–33 %) | |
| Fountain et al, 2021 [27] | Fifteen Neuro-oncology Centres in the UK | Prospective cohort study | One thousand three-hundred and fifty-seven (1357) referrals for newly diagnosed or recurrent intracranial tumours | Patients identified from 1/4/20–31/5/20 with thirty day follow up | 20 % of HGG patients were offered supportive care instead of chemotherapy, 10 % recommended treatment delays |
| Vaandering et al, 2021 [96] | Survey of Twenty-Six Belgian RT Departments, Belgium | Cross-sectional study | Responses received from twenty-one (21) RT departments | Survey started 2/3/20 and re-submitted weekly for four months | Changes in indications for treatment (5 % of centres) and fractionation schemes (15 % of centres), Twenty-four staff acquired COVID-19 |
| He et al, 2021 [43] | Radiation Department, Anhui Provincial Cancer Hospital, China | Retrospective analysis | 29/1/20–11/4/20 vs 9/2/19–23/4/19 | Reduced number of glioblastoma patients receiving RT (17 → 10) and brain metastases (13 → 8). Reduced % composition of Neuro-oncology as indication for RT | |
| Mrugala et al, 2021 [36] | International Survey of twenty-one Neuro-oncology organisations across 6 continents | Cross sectional study | Five hundred and eighty-two (5 8 2) respondents (45 % US, 55 % non-US) across twenty-one (21) organisations | 24/4/20–17/5/20 | 46 % believed temozolomide increases COVID-19 risk, 48.9 % believed steroid use increases COVID-19 risk, 94 % reported changes to practice (highest USA, Europe) and 44.5 % believe this will affect survival. 80 % noticed increased anxiety/depression in patients. 20 % noticed increase in palliative care needs |
Abbreviations: USA – The United States of America; RT – radiation therapy; CNS – central nervous system; UK – The United Kingdom.
Experiences were mixed in paediatric centres (Table 3 ). Some centres had sustained or increased treatment numbers [21], [26], whilst others experienced reductions [34], [39]. One centre had a reduction in number of new brain cancer diagnoses [44]. One centre described telemedicine or email use for the majority of outpatient appointments [45].
Table 3.
Summary of clinical studies investigating the impact of the COVID-19 pandemic on management of paediatric Neuro-oncology patients.
| Author | Centre | Type of Study | Number in study | Time Period | Effect of Pandemic |
|---|---|---|---|---|---|
| Khalafallah et al, 2020 [39] | Department of Neurosurgery, Johns Hopkins University School of Medicine, USA | Retrospective review | Mar 2020 vs Apr 2020 | Number of paediatric neurosurgeries decreased from 15 to 3 | |
| Kilgore et al, 2021 [21] | Ochsner Medical Centre and Tulane Medical Centre, USA | Retrospective review | Mar-Jun 2020 vs equivalent period in 2017–2019 | 233 % increase in paediatric neurosurgeries (low sample size) | |
| Martinez et al, 2020 [57] | Latin American Survey of Radiation Oncologists | Cross-sectional study | Responses from one to twenty-seven (1–27) radiation oncologists per centre from one-hundred and fifteen (1 1 5) RT services | 6/5/20–30/5/20 | Treatment delays for low-grade CNS tumours (11.3 % of centres) |
| Ashkan et al, 2021 [26] | King’s College Hospital NHS Foundation Trust, UK | Prospective cohort study | 18/3/20–15/5/20 vs 18/1/20–17/3/20 | Number of ops. increased in (7 → 9) | |
| Dyson et al, 2020 [122] | Great Ormond Street Hospital for Children NHS Foundation Trust, UK | Prospective observational cohort study | 23/5/20–3/5/20 vs 25/3/19–5/5/5/10 | No delays in referral of time-critical brain tumours | |
| Di Rocco et al, 2020 [45] | Neurochirurgie Pédiatrique, Hôpital Femme-Mère-Enfant, Université de Lyon, France | Letter to the editor | Ops. maintained for CNS tumours (92 % of centres), reduced number of planned ops (20–100 %). 60–90 % of appointments were conducted remotely (via telemedicine or email) | ||
| Kutluk et al, 2021 [44] | Paediatric Oncology Department, Hacettepe University Oncology Hospital, Turkey | Retrospective study | 10/3/20–31/10/2020 vs 10/3/19–31/10/19 | Reduced new CNS cancer diagnoses (39 → 30) | |
| Saab et al, 2020 [58] | Survey of Head of Oncology Units within Paediatric Oncology East and Mediterranean (POEM) group | Cross-sectional study | Surveys collected from thirty-four (34) centres from nineteen (19) countries from Middle East, North Africa and West Asia | Surveys collected between 11/4/20–20/4/2020 | Delays in neurosurgery and radiotherapy (Kuwait most affected). Patients with weekly treatment regimens (LGGs) most affected in Algeria |
| Suryaningtyas et al, 2020 [34] | Surabaya Academic Tertiary Hospital, Indonesia | Retrospective analysis | 1/1/20–14/6/20 vs Apr 2020-future | Reduced number of ops (105 → 44) |
Abbreviations: USA – The United States of Americal; CNS – central nervous system; LGG – low-grade glioma; UK – The United Kingdom.
Similarly, experiences in Neuro-oncological research were variable (Table 4 ). Generally, journals had an increased number of submissions during the pandemic [36], [46], [47], whilst early-phase trials were frequently suspended [4], [36]. A survey of academic practice in neurosurgical centres found that lower-income countries were more likely to access COVID-19 information from less credible sources [48].
Table 4.
Summary of clinical studies investigating the impact of the COVID-19 pandemic on Neuro-oncological research.
| Author | Study | Type of Study | Number in study | Time period | Effect of Pandemic |
|---|---|---|---|---|---|
| Cifarelli et al, 2021 [46] | Journal of Neuro-oncology, (submissions from USA, China, Japan, Germany, Canada, France, Italy, India) | Retrospective review | First two quarters of 2020 compared to equivalent period in 2019 | Increased submissions (+44 %), unchanged proportions of clinical (75 %) to laboratory (25 %) research. All countries had increases in publications (+112 % in Italy) except Japan (–23 %) | |
| El-Ghandour, 2020 [48] | Survey of neurosurgeons from ninety-six countries | Cross-sectional study | Surveys received from six hundred and sixty-one (6 6 1) neurosurgeons (higher proportion in Americas and Europe) | 20/3/20–3/4/20 | 26.7 % of neurosurgeons had a cessation of research. Higher income countries more likely to appreciate the seriousness of the pandemic. Lower-income countries acquired COVID-19 knowledge from less credible sources |
| Lee et al, 2020 [47] | Comparison of neurosurgical and neuro-interventional academic output in eight journals | Retrospective observational study | Month-by-month analysis of submissions from 2016 to 2020 (lockdown period considered March-May 2020) | Increase in submissions during lockdown period for all journals | |
| Mrugala et al, 2021 [36] | International Survey of twenty-one Neuro-oncology organisations across 6 continents | Cross-sectional study | Five hundred and eighty-two (5 8 2) respondents (45 % US, 55 % non-US) | Respondents suspended enrolment for at least 1 clinical trial (67 %), phase I trials (50 %), phase I/II (52 %), 53 phase II (53 %) and phase III (62 %). Respondents closed laboratories (63 %, highest in USA), suspended long-term experiments (72.7 %), had more time to write research (48.7 %) | |
| Simonelli et al, 2020 [4] | Humanitas Cancer Center, Milan; Department of Medical Oncology, Bellaria Hospital, Bologna; and Veneto Institute of Oncology, Padua |
Commentory | Early phase I and II clinical trials suspended |
Abbreviations: USA – The United States of America.
3. Discussion
3.1. General comments
The COVID-19 pandemic has forced clinicians to think imaginatively about solutions in healthcare. Despite the challenges and tragedy experienced by many, innovative solutions to improve healthcare delivery have emerged. The rapid and unprecedented development of the COVID-19 vaccine has involved experts from many fields, and ASCO endorses the use of the Pfizer-BioNTech, Moderna, and Johnson & Johnson/Janssen vaccines in patients with cancer [49]. Additionally, ASCO allows for a fourth dose to be given to select immunocompromised individuals, depending on the context.
One major development during the COVID-19 pandemic involved the use of technology to reduce in-person visits. Telemedicine is designed to reduce traffic in clinical settings; such technology reduces travel requirements, especially relevant for rural and remote patients, as well as patients with neurological deficits [50]. Additionally, telemedicine reduces waiting times, increases flexibility and reduces productivity losses, whilst also positively impacting on patient experience [51], [52], [53], [54], [55], [56], [57], [58]. One centre found that patients who utilised telemedicine appointments had more stable brain tumours than patients who attended in-person appointments [40]. Studies have suggested that telemedicine is suitable for Neuro-oncological examination of non-critical patients [3], [22], [59], [60], reducing the need for in-person visits. Examination may be improved by the presence of a caregiver and appropriate staff training [61]. In support of patient and clinical acceptance, one centre reported that telemedicine appointments did not change after quarantine was lifted [22]. This may reflect continued anxiety about COVID-19 infection or indicate a preference for such technology that persists in the future. Limits of telemedicine include lack of physical examination, technological accessibility and patient preference for in-person visits [50]. There are also concerns that accessibility is a greater issue for racial minorities and people with low education and income [61]. Supporting this observation is a survey of neurosurgeons which found that telemedicine usage was greater in high-income countries, mainly because of access to technology, but also because low-income countries had greater issues with patient education about COVID-19 risks and public health messages [48].
3.2. Impact on Neurosurgery
As the pandemic unfolded, neurosurgeons were forced to reconsider surgical indications. Given the risks of delaying operations, many centres developed a priority system for tumours that were not to be delayed [62], [63], [64], [65], [66], [67]. Cases in Italy and Mexico were classified as either A++ (treat immediately), A+ (surgery within 7–10 days) or A (surgery within one month) [62], [64]. An Italian centre described this as a sustainable approach, especially with reduced traumatic emergencies due to nationwide lockdowns [62]. Generally, HGG surgery was still regarded as urgent, whilst patients with LGGs were managed using a ‘watch and wait’ approach, until more resources became available [15], [68]. Alternatively, the Italian Society of Neurosurgery recommended against delaying surgery for LGGs, due to risk of high grade transformation [59]. For brain metastases, The American Association of Neurological Surgeons, CNS Tumour Section and The Society for Neuro-oncology recommended that surgery only be indicated for large lesions causing mass effect and vasogenic oedema and for patients with a prognosis of greater than three months [15]. Similarly, for spinal metastases, surgery was recommended for progressive deformity, neurological deficits or for significant epidural spinal disease [15]. Some centres viewed all malignant tumours as requiring surgery, with progression of neurological deficit being a factor in determining urgency [22], [52]. Other centres suggested a more case-by-case analysis of indications for surgery. In New York City, a well-publicised COVID-19 hotspot in early 2020, one centre reported a greater consideration of age, comorbidities and overall prognosis in determining the need for surgery. Operations were restricted to those where preservation of neurological function or improvement in prognosis could be achieved [69].
Many centres also questioned the safety of performing high-risk operations during the COVID-19 pandemic. A UK centre performed their first awake craniotomy-nine weeks into the pandemic with a pre-pandemic rate of ∼ 50 per year [70]. These procedures carry inherent risk due to high staff numbers and a risk of sudden conversion to a general anaesthetic. Consequently, the patient was required to self-isolate for fourteen days before the operation. Magnetic resonance imaging confirmed excellent lesional resection without complications and all staff and patients remained COVID-19-negative. Another controversial topic concerns TSS, as close proximity to the nasal mucosa theoretically increases the risk of COVID-19 transmission. Concerns about TSS increased after a patient in China developed COVID-19 disease post-pituitary surgery leading to an outbreak involving 14 staff members (though this was likely post-operative transmission) [71]. Many centres avoided performing these surgeries or converted to a transcranial approach unless required for emergency cases [32], [36], [65], [68], [72], [73], [74], [75], [76], [77]. This concern is shared by the NSA, who have deemed the procedure as involving very significant risk due to high viral shedding from the nasal cavity [78]. Emergencies necessitating TSS include high-flow CSF leaks, pituitary apoplexy, and progressive neurological deficits [66]. Conversely, an Egyptian centre maintains that the endonasal approach is not linked to heightened COVID-19 infection risk [79]. Regardless, recommendations for performing TSS during the COVID-19 pandemic involve pre-operative testing, increased intraoperative irrigation use, mandatory staff PPE and introduction of less-aerosolising rongeurs and chisels [15], [80], [81].
3.3. Impact on delivery of surgical services
Given the increased demands on critical care staff in treating COVID-19 patients, availability of anaesthetic staff became an important consideration in planning neurosurgical treatment. Surveys of critical care doctors across Spain and the UK showed that many anaesthetists suspended perioperative services to treat COVID-19 patients [82], [83]. Several anaesthetists also described anxiety around exposure to COVID-19 and concerns surrounding PPE availability. Another study recounted a case of an anaesthetist who had an episode of dizziness and headache during an operation while wearing PPE, but was unable to doff as he was the only available anaesthetist due to pandemic-related reduced rostering [84]. Additionally, risks to anaesthetists for procedures such as TSS are similar to those faced by neurosurgeons, and anaesthetics staff had to employ measures to combat this [85].
Another important factor in the delivery of surgical services concerns the timing of surgery in patients with current of recent COVID-19 infections. Generally, COVID-19-positive patients were only operated on in cases of absolute emergency, and strict PPE was used by staff [15]. Several centres described poor post-operative outcomes in COVID-19-positive patients. These ranged from hyponatraemia [86], death due to COVID-19-pneumonia [87], [88], transition to palliative care following COVID-19 treatment complications [89] and death due to intracranial haemorrhage post-diagnostic biopsy [90], [91]. The latter cases were patients whose treatment was delayed until they received a negative swab – it remains unclear whether these were false-negatives or if these patients had long-lasting post-infective coagulopathies. Post-operative risk factors for mortality include respiratory failure, development of acute respiratory distress syndrome and dyspnea [92]. Alternatively, successful operations on COVID-19-positive patients have been described. These involve a craniectomy for a solitary cerebellar metastasis [93] and an awake craniotomy for glioblastoma [94]. Both patients were asymptomatic and had no COVID-19 or operation-related complications.
For patients who have recovered from COVID-19, current literature suggests that 30-day postoperative mortality is higher than baseline in the first seven weeks post-viral diagnosis [95]. If possible, operations should be delayed until seven weeks have passed, however this may not be practical for many Neuro-oncology cases. Recommendations are less clear for patients who are still symptomatic after seven weeks, as postoperative mortality remains above baseline.
3.4. Impact on radiation therapy
The majority of glioma patients receive post-operative RT and alkylating therapy regardless of oncological subtype. Although effective, such regimens require regular contact with the healthcare system and thus increase risk of COVID-19 exposure. A survey of Belgian RT departments showed that 18.6 % of staff members contracted COVID-19 at some point, illustrating the concern for staff safety [96]. Thus, many centres introduced patient screening with low threshold for SARS-CoV-2 PCR testing [60]. Interestingly, a positive result in a patient with mild or asymptomatic COVID-19 infection may not preclude treatment, provided the RT centre has appropriate infection control procedures. Evolution into moderate-severe COVID-19 disease indicated pausing of RT until the disease course is over.
RT provides a rather unique opportunity to treat patients in the outpatient setting, reducing the risk of a nosocomial COVID-19 infection [17]. Decisions around treatment alterations were often determined by both oncological and patient factors. In an ideal situation, daily RT for 6 weeks is recommended for WHO Grade 3 and 4 gliomas. Even in the pandemic setting, it is justifiable to continue this optimal treatment in patients younger than 60 [19]. However, this may be unrealistic following outbreaks in RT centres, staff shortages and increased COVID-19 risk. There is more ambiguity in the use of RT in the treatment of elderly, frail and/or LGG patients. Most centres discussed the use of hypofractionation in these settings [15], [19], [59], [97], [98], [99]. For elderly and frail patients with glioblastoma, if RT is an option, hypofractionated therapy is preferred with a dose determined by the COVID-19 risk within the centre’s geographical context [60], [97], [100]. For LGGs, similar to neurosurgical treatment, ‘watch and wait’ scenarios have been adopted, whereby need for RT is determined by clinicoradiological deterioration [15]. Geographical variation and access to radiation therapy likely influence decision-making, with some African RT guidelines recommending complete omission of RT for LGG recurrence [97]. Regarding post-RT medical treatment, clinicians are encouraged to rationalise steroid use, due to the potential for immunosuppression [17]. Overall, oncology teams are encouraged to plan sessions and tests so that all interventions can be performed on as few days as possible, reducing hospital interaction.
3.5. Impact on Medical oncology
Concurrent and adjuvant temozolomide forms part of the standard of care for HGGs, along with maximal safe surgical resection and RT. MGMT-promoter methylation status is a predictive marker for temozolomide sensitivity [99]. As the risk of COVID-19 infection increased, the normal risk–benefit considerations of chemotherapy were reconsidered given temozolomide may lead to myelotoxicity, and lymphopaenia is a risk factor for severe COVID-19 disease [19]. Temozolomide may also cause a re-emergence of COVID-19 infection, even after a negative result has been obtained [86]. In affected patients, haematological toxicity may lead to additional hospital admissions for treatment, further aggrandising infection risk [60]. Such concerns are reflected in a UK study, where 20 % of HGG patients were offered supportive care instead of chemotherapy [27]. ASCO-endorsed guidelines indicate the continued use of temozolomide for MGMT-hypermethylated gliomas, with risk mitigation strategies including reduced steroid dosing and close monitoring of neutrophil and lymphocyte counts [53]. Care should be taken for elderly patients, especially in the high COVID-19 risk setting (infection rate > 5 % and mortality rate > 20 %). In 2019, a Phase III trial indicated increased survival when temozolomide is used in combination with lomustine for MGMT-hypermethylated glioblastoma [101]. Due to lomustine-induced haematological side-effects, however, this regime has been discouraged during the pandemic [19]. For MGMT-unmethylated gliomas, especially in the elderly group, ASCO-endorsed guidelines have advised a case-by-case evaluation [53], whilst other centres have advised against temozolomide use due to lacking supportive evidence [4], [60], [100]. Hypofractionated RT alone may be a better option for elderly patients, with early data suggesting reduced adverse events and death compared to temozolomide [100]. A recent study has suggested benefit from TTFs where survival in elderly glioblastoma patients was greater with combination TTFs/temozolomide therapy compared to temozolomide alone, without any change in toxicity profile [102]. Although not offered in the Australian setting, TTFs may present a safe option as treatment can occur remotely in the patient’s home. Regardless, the development of a clear medical oncology plan remains important during the pandemic. This was made evident by a Wuhan study, where clinicians hypothesised that an increased recurrence of CNS tumours resected pre-pandemic reflected reduced access to conventional chemotherapeutic agents due to COVID-19 concerns [103].
Best evidence adjuvant medical treatment for LGGs remains controversial, with pre-pandemic uncertainty regarding whether temozolomide or a combination of procarbazine, CCNU (lomustine) and vincristine (PCV) has a stronger rationale [104]. Local guidelines indicate that when used in WHO Grade 2 and 3 disease, PCV treatment is associated with low lymphopaenia risk and causes few clinically significant infections [53]. However, haematological toxicity of vincristine and pulmonary fibrosis risk of procarbazine and lomustine make this regimen risky in the COVID-19 environment [19]. Previous guidelines for oligodendroglial tumours have advised RT with adjuvant PCV, however during the pandemic centres have either omitted vincristine or switched PCV to temozolomide [19].
The pandemic has influenced the use of second/third line systemic therapy where there is limited evidence for survival benefit in patients. For recurrent glioblastoma, ASCO-endorsed guidelines suggest that bevacizumab is preferable to lomustine as it lacks myelotoxicity and reduces steroid use [53]. Concern has also arisen around supportive care, where high dose steroids are often used for CNS tumours to reduce cerebral oedema. The lowest dose possible, 10 mg, should be used to maintain anti-inflammatory effect without immunosuppression. If high doses are required, patients are advised to take trimethoprim and sulfamethoxazole to reduce the risk of interstitial pneumonia, and a low-dose diuretic [4]. Regarding patients who decide against aggressive treatment, clinicians should devise a supportive and palliative care plan that avoids emergency and inpatient hospital admissions [60].
3.6. Impact on paediatric Neuro-oncology
The COVID-19 pandemic has created unique considerations for the practice of paediatric Neuro-oncology. In comparison to adults, children tend to develop milder symptoms secondary to COVID-19 infection [105], [106], [107]. A UK study has also shown that children with cancer have a similar risk of developing severe COVID-19 disease compared to healthy children [108]. Whilst risk to healthcare workers and adult family remains high, it is theoretically reasonable to maintain treatment for paediatric tumours during the pandemic. For example, treatment in Australia is recommended to continue with strict isolation precautions [109]. However, treatment delays were still observed across the globe. One study described a reduction in new diagnosis of paediatric CNS-related cancer [44], with uncertain implications for future presentations. A Rome Paediatric Oncology Department treated four children who presented with clinical decompensation due to brain cancer, which was described as four times the usual number [110]. All children had been seen previously by a paediatrician, however their treatment had been deferred due to the pandemic. Interestingly, some clinicians have suggested that school closures during lockdown have contributed to delayed presentations, as teachers are often the first to notice symptoms. This is important to consider as delayed presentations are associated with worse outcomes [107]. Brazilian guidelines suggest that children presenting with tumour-related mass effect require emergency surgery, whilst all other new CNS tumours should be treated within three days. Conditions that can be postponed include stable tumour recurrences, endonasal operations and radiosurgery [45], [72]. Similar views are held in France [45]. One paper describes the need to treat paediatric tumours without hydrocephalus or mass effect, due to the risk of haemorrhage, progression to hydrocephalus or de-differentiation into a higher-grade lesion [111]. Additionally, clinicians have noticed mental health deterioration within paediatric Neuro-oncology populations. A USA survey found that compared to pre-pandemic times, survivors had negatively affected life satisfaction and social connectedness, both being predictors for the patient developing post-traumatic stress [112]. A Canadian survey of medulloblastoma patients found that children experienced increased social isolation from friends [113]. Conversely, both studies did report some positives during the pandemic. These included positive parenting changes and increased family cohesion.
3.7. Impact on Neuro-oncological research
Research remains a very important part of Neuro-oncology given the poor prognosis of many CNS tumour patients. Clinical trials provide hope and treatment opportunities for patients that otherwise have no standard proven therapy. During the pandemic, however, maintenance of research has proven difficult as COVID-19 research became the highest priority. Consequently, Neuro-oncology departments experienced relocation of their funding to laboratories performing COVID-19 research [5]. For continuing studies, however, clinicians were forced to question the ethics of increasing patient visits and providing unknown treatments during the pandemic. The latter is particularly important in oncology, where trials often incorporate the use of immunosuppressive agents. In response to these considerations, the U.S. Food and Drug Administration increased the flexibility of clinical trial guidelines to reduce the risks of nosocomial COVID-19 infection [60]. Phase I trials were generally deemed inappropriate during the pandemic, as these often require frequent visits and use drugs or doses with unknown toxic profiles. Phase II and III trials were continued with caution, modified to increase COVID-19-safe behaviour [99]. Late-stage trials were prioritised using decentralisation, where treatment, imaging and pathology is completed in the community where possible [61]. Suitable trials for decentralisation were those with less-subjective measurements such as survivability and trials with interventions that can be completed at home, such as TTFs [102]. Trials unsuitable for decentralisation include those that require specialist monitoring and complex facilities, such as paediatric brain tumour patients. As a rule, Neuro-oncology patients previously on trials should continue regimes to avoid a lapse in care [15]. Expert opinion suggests that current or previous COVID-19 infection should not exclude patients from enrolling in clinical studies [61]. Regarding non-therapeutic research, such as research for natural disease history, supportive care, and quality of life, studies only continued remotely during the pandemic. Non-therapeutic programs that required visits were cancelled altogether, such as pharmacokinetic blood analyses and on-site non-therapeutic biopsies. For cancelled studies, clinicians worked with statisticians to maintain the validity of prior data with sudden changes in sample sizes. Many centres also encouraged the maintenance of journal club sessions, made possible with video technology [64].
Whilst currently speculative, the above impacts may have irreversible implications on the field of Neuro-oncological research. Firstly, it is foreseeable that some cancelled trials may never be restarted due to funding issues and pursuit of new treatments. The value of such therapies will never be realised, and it will be near impossible to determine the true extent of this. Secondly, for trials that did continue, it will be very difficult to interpret the clinical trial data gained during the pandemic. Drug development requires timely delivery of medicine, pathology sample collection and consistent patient clinical evaluation – all of these factors were impacted by the COVID-19 pandemic [114]. For novel therapies that did fail, clinicians will be unsure if this truly reflects the treatment, or if this represents a pandemic-related treatment limitation. Lastly, due to the length of the COVID-19 pandemic, it is plausible that there will be a delay in the approval of novel Neuro-oncology therapies. Considering the short life-expectancies associated with many CNS cancers, this could dramatically reduce available treatment options, and favour an earlier transition to end-of-life care.
4. Conclusion
The COVID-19 pandemic has forced a re-evaluation of clinical practice across the globe, and Neuro-oncology has been no exception. Neuro-oncology centres worldwide have seen reductions in patient referrals, treatment numbers and new diagnoses during this pandemic. As risk to patients and staff remain high, staff protocols and treatment guidelines have adapted to ensure the ongoing provision of safe care for this highly vulnerable patient group.
5. Recommendations
The following modifications aided Neuro-oncology centres in continuing safe care during the COVID-19 pandemic:
-
a)
Early establishment of triage criteria, where urgent care is maintained while non-urgent care is postponed or withdrawn altogether
-
b)
Alteration of treatment options to reduce in-person healthcare system interactions (e.g. practice hypofractionated radiotherapy; avoidance of myelotoxicity; use of telemedicine)
-
c)
Resource allocation methods in Neuro-oncology management decision-making (i.e. availability of post-operative ICU beds)
-
d)
Rigorous application of the principles of screening, PPE, social distancing and contact tracing
-
e)
Regular monitoring of local epidemiological status to adjust treatment plans based on infection risk
-
f)
Improved safety protocols in operating rooms for neurosurgical and TSS procedures
-
g)
Where safe, delay surgical management of Neuro-oncology patients with recent COVID-19 infections to ≥ 7 weeks from viral diagnosis.
Funding
No funding was received for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arimappamagan A., Vilanilam G., Pandey P. Is Elective Neurosurgery Justified During COVID-19 Pandemic? Neurology India. 2021;69(1) doi: 10.4103/0028-3886.310113. [DOI] [PubMed] [Google Scholar]
- 3.Raffiq A., Seng L.B., San L.S., Zakaria Z., Yee A.S., Fitzrol D.N., et al. COVID-19 Pandemic and Its Impact on Neurosurgery Practice in Malaysia: Academic Insights, Clinical Experience and Protocols from March till August 2020. Malays J Med Sci. 2020;27(5):141–195. doi: 10.21315/mjms2020.27.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonelli M., Franceschi E., Lombardi G. Neuro-Oncology During the COVID-19 Outbreak: A Hopeful Perspective at the End of the Italian Crisis. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.594610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amidei C., Arzbaecher J., Maher M.E., Mungoshi C., Cashman R., Farrimond S., et al. The brain tumor not-for-profit and charity experience of COVID-19: reacting and adjusting to an unprecedented global pandemic in the 21st century. Neurooncol Adv. 2021;3(1) doi: 10.1093/noajnl/vdaa166. vdaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.School of Geography UoM. The Geographies of COVID-192020; (11/3/2022). Available from: https://pursuit.unimelb.edu.au/articles/the-geographies-of-covid-19.
- 7.OECD. The territorial impact of COVID-19: Managing the crisis and recovery across levels of government2021. Available from: https://www.oecd.org/coronavirus/policy-responses/the-territorial-impact-of-covid-19-managing-the-crisis-and-recovery-across-levels-of-government-a2c6abaf/.
- 8.Cook M.J., Dri G.G., Logan P., Tan J.B., Flahault A. COVID-19 Down Under: Australia's Initial Pandemic Experience. Int J Environ Res Public Health. 2020;17(23) doi: 10.3390/ijerph17238939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desborough J., Hall Dykgraaf S., Davis S., Kidd M. Reflecting on Australia's five principles for pandemic response in primary care through the lens of early international experiences of COVID-19. Aust. J Gen Pract. 2021 doi: 10.31128/AJGP-COVID-46. [DOI] [PubMed] [Google Scholar]
- 10.BBC News. 2021 [Google Scholar]
- 11.Taylor A., Caffery L.J., Gesesew H.A., King A., Bassal A.R., Ford K., et al. How Australian Health Care Services Adapted to Telehealth During the COVID-19 Pandemic: A Survey of Telehealth Professionals. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.648009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson R., Mian M., Sreedharan S., Lau P. The Impact of COVID-19 on First Nations People Health Assessments in Australia. Asia Pac J Public Health. 2021;33(5):595–597. doi: 10.1177/10105395211011012. [DOI] [PubMed] [Google Scholar]
- 13.Grey A. Australia's multilingual communities are missing out on vital coronavirus information. ABC News. 2020 29/6/2020. [Google Scholar]
- 14.The Royal Australian College of General Practitioners. Vaccination gap: Vulnerable communities left exposed as Omicron threatens2022. Available from: https://www1.racgp.org.au/newsgp/clinical/vaccination-gap-vulnerable-communities-left-expose.
- 15.Ramakrishna R., Zadeh G., Sheehan J.P., Aghi M.K. Inpatient and outpatient case prioritization for patients with neuro-oncologic disease amid the COVID-19 pandemic: general guidance for neuro-oncology practitioners from the AANS/CNS Tumor Section and Society for Neuro-Oncology. J Neurooncol. 2020;147(3):525–529. doi: 10.1007/s11060-020-03488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neurosurgical Society of Australasia. COVID-19 - Neurosurgical Society of Australasia. 2021.
- 17.Pannullo S.C., Chidambaram S., Brandmaier A., Knisely J., Adler J.R., Jr. Clinical Considerations in Neurosurgical Radiosurgery in the Time of COVID-19. Cureus. 2020;12(4) doi: 10.7759/cureus.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean W.C., Ironside N.T., Sack K.D., Felbaum D.R., Syed H.R. The impact of COVID-19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study. Acta Neurochir (Wien) 2020;162(6):1229–1240. doi: 10.1007/s00701-020-04342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernhardt D., Wick W., Weiss S.E., Sahgal A., Lo S.S., Suh J.H., et al. Neuro-oncology Management During the COVID-19 Pandemic With a Focus on WHO Grade III and IV Gliomas. Neuro Oncol. 2020 doi: 10.1093/neuonc/noaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Society of Clinical Oncology. Cancer Treatment & Supportive Care 2022 [Available from: https://www.asco.org/covid-resources/patient-care-info/cancer-treatment-supportive-care.
- 21.Kilgore M.D., Scullen T., Mathkour M., Dindial R., Carr C., Zeoli T., et al. Effects of the COVID-19 Pandemic on Operative Volume and Residency Training at Two Academic Neurosurgery Centers in New Orleans. World Neurosurgery. 2021;151:e68–e77. doi: 10.1016/j.wneu.2021.03.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luther E., Burks J., Eichberg D.G., Basil G., Berry K., Lu V., et al. Neuro-oncology practice guidelines from a high-volume surgeon at the COVID-19 epicenter. J Clin Neurosci. 2021;85:1–5. doi: 10.1016/j.jocn.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad H., Alawieh A., Oyesiku N., Barrow D.L., Olson J. Sheltered Neurosurgery During COVID-19: The Emory Experience. World Neurosurg. 2020;144:e204–e209. doi: 10.1016/j.wneu.2020.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wali A.R., Ryba B.E., Kang K., Santiago-Dieppa D.R., Steinberg J., Diaz-Aguilar L.D., et al. Impact of COVID-19 on a Neurosurgical Service: Lessons from the University of California San Diego. World Neurosurg. 2021;148:e172–e181. doi: 10.1016/j.wneu.2020.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja S., Shah P., Mohammed R. Impact of COVID-19 pandemic on acute spine surgery referrals to UK tertiary spinal unit: any lessons to be learnt? Br J Neurosurg. 2021;35:181–185. doi: 10.1080/02688697.2020.1777263. [DOI] [PubMed] [Google Scholar]
- 26.Ashkan K., Jung J., Velicu A.M., Raslan A., Faruque M., Kulkarni P., et al. Neurosurgery and coronavirus: impact and challenges-lessons learnt from the first wave of a global pandemic. Acta Neurochir (Wien) 2021;163(2):317–329. doi: 10.1007/s00701-020-04652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fountain D.M., Piper R.J., Poon M.T.C., Solomou G., Brennan P.M., Chowdhury Y.A., et al. CovidNeuroOnc: A UK multicenter, prospective cohort study of the impact of the COVID-19 pandemic on the neuro-oncology service. Neurooncol Adv. 2021;3(1) doi: 10.1093/noajnl/vdab014. vdab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price S.J., Joannides A., Plaha P., Afshari F.T., Albanese E., Barua N.U., et al. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: a national survey (COVID-CNSMDT Study) BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dannhoff G., Cebula H., Chibbaro S., Ganau M., Todeschi J., Mallereau C.H., et al. Investigating the real impact of COVID-19 pandemic on the daily neurosurgical practice? Neurochirurgie. 2021;67(2):99–103. doi: 10.1016/j.neuchi.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosravi M.H., Sisakht A.M., Kiani D., Ahmadi S. Letter to the Editor “Effects of Coronavirus Disease 2019 (COVID-19) Pandemic on Neurological Surgery Care and Education; Our Experience from Iran”. World Neurosurg. 2020;139:376. doi: 10.1016/j.wneu.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hameed N.U.F., Ma Y., Zhen Z., Wu S., Feng R., Li W., et al. Impact of a pandemic on surgical neuro-oncology-maintaining functionality in the early phase of crisis. BMC Surg. 2021;21(1):40. doi: 10.1186/s12893-021-01055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal N., Venkataram T., Singh V., Chaturvedi J. Collateral damage caused by COVID-19: Change in volume and spectrum of neurosurgery patients. J Clin Neurosci. 2020;80:156–161. doi: 10.1016/j.jocn.2020.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manusubroto W., Wicaksono A.S., Tamba D.A., Sudiharto P., Pramusinto H., Hartanto R.A., et al. Neurosurgery Services in Dr. Sardjito General Hospital, Yogyakarta, Indonesia, During the COVID-19 Pandemic: Experience from a Developing Country. World Neurosurgery. 2020;140:e360–e366. doi: 10.1016/j.wneu.2020.05.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suryaningtyas W., Wahyuhadi J., Turchan A., Subagio E.A., Parenrengi M.A., Apriawan T., et al. Neurosurgery at the epicenter of the COVID-19 pandemic in Indonesia: experience from a Surabaya academic tertiary hospital. Neurosurg Focus. 2020;49(6):E5. doi: 10.3171/2020.9.FOCUS20559. [DOI] [PubMed] [Google Scholar]
- 35.Antony J., James W.T., Neriamparambil A.J., Barot D.D., Withers T. An Australian Response to the COVID-19 Pandemic and Its Implications on the Practice of Neurosurgery. World Neurosurg. 2020;139:e864–e871. doi: 10.1016/j.wneu.2020.05.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mrugala M.M., Ostrom Q.T., Pressley S.M., Taylor J.W., Thomas A.A., Wefel J.S., et al. The state of neuro-oncology during the COVID-19 pandemic: a worldwide assessment. Neurooncol Adv. 2021;3(1) doi: 10.1093/noajnl/vdab035. vdab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahrou F., M'Barek Y.A., Belokda T., Drai B., Abdourafiq H., Benantar L., et al. Will the high acceptance rate of coronavirus disease 2019 vaccine in Morocco accelerate the recovery of neurosurgical practice? Surg Neurol Int. 2021;12:486. doi: 10.25259/SNI_690_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson G.E., Islim A.I., Albanese E., Ahmed A., Aly A., Ammar A., et al. Neurosurgery activity levels in the United Kingdom and republic of Ireland during the first wave of the covid-19 pandemic - a retrospective cross-sectional cohort study. Br J Neurosurg. 2021;1–6 doi: 10.1080/02688697.2021.1968341. [DOI] [PubMed] [Google Scholar]
- 39.Khalafallah A.M., Jimenez A.E., Lee R.P., Weingart J.D., Theodore N., Cohen A.R., et al. Impact of COVID-19 on an Academic Neurosurgery Department: The Johns Hopkins Experience. World Neurosurgery. 2020;139:e877–e884. doi: 10.1016/j.wneu.2020.05.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman S., Ramos A., Giantini Larsen A.M., Bander E., Goldberg J., Parker W., et al. Impact of the COVID-19 pandemic on neuro-oncology outcomes. J Neurooncol. 2021;154(3):375–381. doi: 10.1007/s11060-021-03838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer K., Jones C.M., Girdler R., Roe C., Sharpe M., Lawton S., et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. 2021;22(3):309–320. doi: 10.1016/S1470-2045(20)30743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberge D., Delouya G., Bohigas A., Michalowski S. Catching the Wave: Quantifying the Impact of COVID on Radiotherapy Delivery. Curr Oncol. 2020;28(1):152–158. doi: 10.3390/curroncol28010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J., Yang L., Tao Z., Yang J., Zhou Y., Wang R., et al. Impact of the 2019 Novel Coronavirus Disease (COVID-19) Epidemic on Radiotherapy-Treated Patients with Cancer: A Single-Center Descriptive Study. Cancer Manag Res. 2021;13:37–43. doi: 10.2147/CMAR.S281323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kutluk M.T., Ahmed F., Kirazli M., Bajin I.Y., Mungen E., Ekinci S., et al. The effect of the COVID-19 pandemic on paediatric cancer care: lessons learnt from a major paediatric oncology department in Turkey. Ecancermedicalscience. 2021;15:1172. doi: 10.3332/ecancer.2021.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Rocco F., Scavarda D., Vinchon M., Szathmari A., Riffaud L., Bohra A., et al. Impact of the COVID-19 pandemic on pediatric neurosurgery in France. [French] Neurochirurgie. 2020;66(4):192–194. doi: 10.1016/j.neuchi.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cifarelli C.P., Sheehan J.P. COVID-19 effects on neuro-oncology publishing: preliminary outcomes & future impacts. J Neurooncol. 2020;149(3):555–556. doi: 10.1007/s11060-020-03621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J.E., Mohanty A., Albuquerque F.C., Couldwell W.T., Levy E.I., Benzel E.C., et al. Trends in academic productivity in the COVID-19 era: analysis of neurosurgical, stroke neurology, and neurointerventional literature. J Neurointerv Surg. 2020;12(11):1049–1052. doi: 10.1136/neurintsurg-2020-016710. [DOI] [PubMed] [Google Scholar]
- 48.El-Ghandour N.M.F., Elsebaie E.H., Salem A.A., Alkhamees A.F., Zaazoue M.A., Fouda M.A., et al. Letter: The Impact of the Coronavirus (COVID-19) Pandemic on Neurosurgeons Worldwide. Neurosurgery. 2020;87(2):E250–E257. doi: 10.1093/neuros/nyaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Society of Clinical Oncology. COVID-19 Vaccines & Patients with Cancer 2022 [Available from: https://www.asco.org/covid-resources/vaccines-patients-cancer.
- 50.Daggubati L.C., Eichberg D.G., Ivan M.E., Hanft S., Mansouri A., Komotar R.J., et al. Telemedicine for Outpatient Neurosurgical Oncology Care: Lessons Learned for the Future During the COVID-19 Pandemic. World Neurosurg. 2020;139:e859–e863. doi: 10.1016/j.wneu.2020.05.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung C, Wadhwa H, Sklar M, Sheth K, Loo S, Ratliff J, et al. Telehealth Adoption Across Neurosurgical Subspecialties at a Single Academic Institution During the COVID-19 Pandemic. [DOI] [PMC free article] [PubMed]
- 52.Raheja A., Agarwal N., Mohapatra S., Tandon V., Borkar S.A., Chandra P.S., et al. Preparedness and guidelines for neurosurgery in the COVID-19 era: Indian perspective from a tertiary care referral hospital. Neurosurg Focus. 2020;49(6):E3. doi: 10.3171/2020.9.FOCUS20564. [DOI] [PubMed] [Google Scholar]
- 53.Segelov E., Underhill C., Prenen H., Karapetis C., Jackson C., Nott L., et al. Practical Considerations for Treating Patients With Cancer in the COVID-19 Pandemic. JCO Oncol Pract. 2020;16(8):467–482. doi: 10.1200/OP.20.00229. [DOI] [PubMed] [Google Scholar]
- 54.Fonkem E., Gatson N.T.N., Tadipatri R., Cole S., Azadi A., Sanchez M., et al. Telemedicine review in neuro-oncology: comparative experiential analysis for Barrow Neurological Institute and Geisinger Health during the 2020 COVID-19 pandemic. Neurooncol Pract. 2021;8(2):109–116. doi: 10.1093/nop/npaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noureldine M.H.A., Pressman E., Krafft P.R., Greenberg M.S., Agazzi S., van Loveren H., et al. Impact of the COVID-19 Pandemic on Neurosurgical Practice at an Academic Tertiary Referral Center: A Comparative Study. World Neurosurgery. 2020;139:e872–e876. doi: 10.1016/j.wneu.2020.05.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soriano Sanchez J.A., Perilla Cepeda T.A., Zenteno M., Campero A., Yampolsky C., Varela M.L., et al. Early Report on the Impact of COVID-19 Outbreak in Neurosurgical Practice Among Members of the Latin American Federation of Neurosurgical Societies. World Neurosurgery. 2020;140:e195–e202. doi: 10.1016/j.wneu.2020.04.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez D., Sarria G.J., Wakefield D., Flores C., Malhotra S., Li B., et al. COVID's Impact on Radiation Oncology: A Latin American Survey Study. Int J Radiat Oncol Biol Phys. 2020;108(2):374–378. doi: 10.1016/j.ijrobp.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saab R., Obeid A., Gachi F., Boudiaf H., Sargsyan L., Al-Saad K., et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on pediatric oncology care in the Middle East, North Africa, and West Asia region: A report from the Pediatric Oncology East and Mediterranean (POEM) group. Cancer. 2020;126(18):4235–4245. doi: 10.1002/cncr.33075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angileri F.F., Sabatino G., Cavallo L.M., Pessina F., Ius T., O ded,, et al. Natura non facit saltus: a phase 2 proposal to manage brain tumors cases from the Neuro-oncology section of the Italian Society of Neurosurgery (SINch(R)) J Neurosurg Sci. 2021;65(1):1–7. doi: 10.23736/S0390-5616.20.05054-7. [DOI] [PubMed] [Google Scholar]
- 60.Mohile N.A., Blakeley J.O., Gatson N.T.N., Hottinger A.F., Lassman A.B., Ney D.E., et al. Urgent Considerations for the Neuro-oncologic Treatment of Patients with Gliomas During the COVID-19 Pandemic. Neuro Oncol. 2020;11:11. doi: 10.1093/neuonc/noaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee E.Q., Selig W., Meehan C., Bacha J., Barone A., Bloomquist E., et al. Report of National Brain Tumor Society roundtable workshop on innovating brain tumor clinical trials: building on lessons learned from COVID-19 experience. Neuro Oncol. 2021;23(8):1252–1260. doi: 10.1093/neuonc/noab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoia C., Bongetta D., Veiceschi P., Cenzato M., Di Meco F., Locatelli D., et al. Neurosurgery during the COVID-19 pandemic: update from Lombardy, northern Italy. Acta Neurochir (Wien) 2020;162(6):1221–1222. doi: 10.1007/s00701-020-04305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ceraudo M., Balestrino A., Cama A., Macrina G., Piatelli G., Consales A. Pediatric Neurosurgery After the COVID-19 Pandemic: Management Strategies from a Single Pediatric Hospital in Italy. World Neurosurg. 2021;146:e1079–e1082. doi: 10.1016/j.wneu.2020.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diaz-Bello S., Hernandez-Hernandez A., Guinto-Nishimura G.Y., Mondragon-Soto M.G., Lem-Carrillo M., Gonzalez-Aguilar A., et al. Reconversion of neurosurgical practice in times of the SARS-CoV-2 pandemic: a narrative review of the literature and guideline implementation in a Mexican neurosurgical referral center. Neurosurg Focus. 2020;49(6):E4. doi: 10.3171/2020.9.FOCUS20553. [DOI] [PubMed] [Google Scholar]
- 65.Sadhasivam S., Arora R.K., Rekapalli R., Chaturvedi J., Goyal N., Bhargava P., et al. A Systematic Review on the Impact of the COVID-19 Pandemic on Neurosurgical Practice and Indian Perspective. Asian J Neurosurg. 2021;16(1):24–32. doi: 10.4103/ajns.AJNS_379_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zacharia B.E., Eichberg D.G., Ivan M.E., Hanft S., Boockvar J.A., Isildak H., et al. Letter: Surgical Management of Brain Tumor Patients in the COVID-19 Era. Neurosurgery. 2020;87(2):E197–E200. doi: 10.1093/neuros/nyaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajunaid K., Alatar A., Alqurashi A., Alkutbi M., Alzahrani A.H., Sabbagh A.J., et al. The longitudinal impact of COVID-19 pandemic on neurosurgical practice. Clin Neurol Neurosurg. 2020;198 doi: 10.1016/j.clineuro.2020.106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozoner B., Gungor A., Hasanov T., Toktas Z.O., Kilic T. Neurosurgical Practice During Coronavirus Disease 2019 (COVID-19) Pandemic. World Neurosurgery. 2020;140:198–207. doi: 10.1016/j.wneu.2020.05.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ammar A., Stock A.D., Holland R., Gelfand Y., Altschul D. Managing a Specialty Service During the COVID-19 Crisis: Lessons From a New York City Health System. Acad Med. 2020;95(10):1495–1498. doi: 10.1097/ACM.0000000000003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Razak A., Sloan G., Sebastian J., Ehsan S., Karabatsou K. Awake craniotomy in the COVID-19 era - technical tips and feasibility. J Clin Neurosci. 2020;82(Pt A):49–51. doi: 10.1016/j.jocn.2020.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu W., Huang X., Zhao H., Jiang X. A COVID-19 Patient Who Underwent Endonasal Endoscopic Pituitary Adenoma Resection: A Case Report. Neurosurgery. 2020;87(2):E140–E146. doi: 10.1093/neuros/nyaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ballestero M.F.M., Furlanetti L., de Oliveira R.S. Pediatric neurosurgery during the COVID-19 pandemic: update and recommendations from the Brazilian Society of Pediatric Neurosurgery. Neurosurg Focus. 2020;49(6):E2. doi: 10.3171/2020.9.FOCUS20703. [DOI] [PubMed] [Google Scholar]
- 73.Alimohammadi E., Eden S.V., Anand S.K., Ahadi P., Bostani A., Bagheri S.R., et al. (COVID-19) on neurosurgical practice and training: a review article. Br J Neurosurg. 2019;2021:1–5. doi: 10.1080/02688697.2021.1888874. [DOI] [PubMed] [Google Scholar]
- 74.Fleseriu M., Dekkers O.M., Karavitaki N. Endocrinology in the time of COVID-19: Management of pituitary tumours. Eur J Endocrinol. 2020;183(1):G17–G23. doi: 10.1530/EJE-20-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell R.A., King J.A.J., Goldschlager T., Wang Y.Y. Impact of COVID-19 on pituitary surgery. ANZ J Surg. 2020;90(6):963–964. doi: 10.1111/ans.15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsermoulas G., Zisakis A., Flint G., Belli A. Challenges to Neurosurgery During the Coronavirus Disease 2019 (COVID-19) Pandemic. World Neurosurgery. 2020;139:519–525. doi: 10.1016/j.wneu.2020.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iorio-Morin C., Hodaie M., Sarica C., Dea N., Westwick H.J., Christie S.D., et al. Letter: The Risk of COVID-19 Infection During Neurosurgical Procedures: A Review of Severe Acute Respiratory Distress Syndrome Coronavirus 2 (SARS-CoV-2) Modes of Transmission and Proposed Neurosurgery-Specific Measures for Mitigation. Neurosurgery. 2020;87(2):E178–E185. doi: 10.1093/neuros/nyaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Position Statement: COVID-19. 2020 [Google Scholar]
- 79.Arnaout M.M., Bessar A.A., Elnashar I., Abaza H., Makia M. Pituitary adenoma surgeries in COVID-19 era: Early local experience from Egypt. Surg Neurol Int. 2020;11:363. doi: 10.25259/SNI_472_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fleseriu M., Buchfelder M., Cetas J.S., Fazeli P.K., Mallea-Gil S.M., Gurnell M., et al. Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic-an international perspective. Pituitary. 2020;23(4):327–337. doi: 10.1007/s11102-020-01059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S.H., Jang J.S., Chung J.W., Kwon J.T., Park Y.S. Clinical Pathway for Emergency Brain Surgery during COVID-19 Pandemic and Its Impact on Clinical Outcomes. J Korean Med Sci. 2021;36(2) doi: 10.3346/jkms.2021.36.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aisa I., Llau J., Gonzalez J.M., Delgado C., Otero M., Romero C.S., et al. Impact of COVID-19 Pandemic on Anesthesia and Critical Care Residents in Spain. Anesth Pain Med. 2021;11(4) doi: 10.5812/aapm.116836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kursumovic E., Cook T.M., Vindrola-Padros C., Kane A.D., Armstrong R.A., Waite O., et al. The impact of COVID-19 on anaesthesia and critical care services in the UK: a serial service evaluation. Anaesthesia. 2021;76(9):1167–1175. doi: 10.1111/anae.15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yunus M., Deb P., Das R., Bhattacharyya P. Significant physiological impact of wearing PPE inside operation theatre: A challenging scenario in this COVID-19 pandemic. J Family Med Prim Care. 2021;10(1):561–563. doi: 10.4103/jfmpc.jfmpc_1711_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flexman A.M., Abcejo A.S., Avitsian R., De Sloovere V., Highton D., Juul N., et al. Neuroanesthesia Practice During the COVID-19 Pandemic: Recommendations From Society for Neuroscience in Anesthesiology and Critical Care (SNACC) J Neurosurg Anesthesiol. 2020;32(3):202–209. doi: 10.1097/ANA.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiore G., Remore L.G., Tariciotti L., Carrabba G.G., Schisano L., Pluderi M., et al. Does COVID-19 Affect Survival and Functional Outcome in Emergency and Urgent Neurosurgical Procedures? A Single-Center Prospective Experience During the Pandemic. World Neurosurgery. 2021 doi: 10.1016/j.wneu.2021.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borsa S., Bertani G., Gay L., Pirola E., Riva M., Caroli M. Call of duty: neuro-oncology outpatient management during the COVID-19 pandemic in Milan. Italy Neuro Oncol. 2020;22(12):1891–1892. doi: 10.1093/neuonc/noaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farahbakhsh F., Rostami M., Khoshnevisan A., Naderian N., Ghorbani M., Fehlings M.G., et al. The Management and Outcomes of Coronavirus Disease 2019 Infection in a Series of Neurosurgical Patients. Asian J Neurosurg. 2021;16(1):78–83. doi: 10.4103/ajns.AJNS_187_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarwan G., Mubarak T., Puello P., Brisman M., Grewal J. Negative Impact of COVID-19 Upon Primary Brain Tumor Care. Cureus. 2021;13(9) doi: 10.7759/cureus.17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lubansu A., Assamadi M., Barrit S., Dembour V., Yao G., El Hadwe S., et al. COVID-19 Impact on Neurosurgical Practice: Lockdown Attitude and Experience of a European Academic Center. World Neurosurgery. 2020;144:e380–e388. doi: 10.1016/j.wneu.2020.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lubansu A., El Hadwe S. In Reply to the Letter to the Editor Regarding “COVID-19 Impact on Neurosurgical Practice: Lockdown Attitude and Experience of a European Academic Center”. World Neurosurgery. 2021;148:224. doi: 10.1016/j.wneu.2020.10.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang K., Wu C., Xu J., Zhang B., Zhang X., Gao Z., et al. Factors affecting the mortality of patients with COVID-19 undergoing surgery and the safety of medical staff: A systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lapolla P., Familiari P., Bruzzaniti P., Arcese R., Matassa R., Frati A., et al. First-in-man craniectomy and asportation of solitary cerebellar metastasis in COVID-19 patient: A case report. Int J Surg Case Rep. 2020;77:753–758. doi: 10.1016/j.ijscr.2020.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okunlola A.I. Awake craniotomy in a Covid-19 positive patient: The challenges and outcome. Interdiscip Neurosurg. 2021;24 doi: 10.1016/j.inat.2020.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lobo D., Devys J.M. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2022;77(1):110. doi: 10.1111/anae.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaandering A., Ben Mustapha S., Lambrecht M., Van Gestel D., Veldmeman L. Impact of the COVID-19 Pandemic on Patients and Staff in Radiation Oncology Departments in Belgium: A National Survey. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.654086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kochbati L., Vanderpuye V., Moujahed R., Rejeb M.B., Naimi Z., Olasinde T. Cancer care and COVID-19: tailoring recommendations for the African radiation oncology context. Ecancermedicalscience. 2020(14):1144. doi: 10.3332/ecancer.2020.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pendyala P., Goglia A.G., Mattes M.D., Grann A., Huang D., Wagman R.T., et al. Impact of the Coronavirus Disease of 2019 Pandemic on Radiation Oncology Clinical Decision Making in a High-Prevalence Environment. Adv Radiat Oncol. 2021;6(3) doi: 10.1016/j.adro.2021.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weller M., Preusser M. How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open. 2020 doi: 10.1136/esmoopen-2020-000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tabrizi S., Trippa L., Cagney D., Tanguturi S., Ventz S., Fell G., et al. A Quantitative Framework for Modeling COVID-19 Risk During Adjuvant Therapy Using Published Randomized Trials of Glioblastoma in the Elderly. Neuro Oncol. 2020;27:27. doi: 10.1093/neuonc/noaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herrlinger U., Tzaridis T., Mack F., Steinbach J.P., Schlegel U., Sabel M., et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. doi: 10.1016/S0140-6736(18)31791-4. [DOI] [PubMed] [Google Scholar]
- 102.Gatson N.T.N., Barnholtz-Sloan J., Drappatz J., Henriksson R., Hottinger A.F., Hinoul P., et al. Tumor Treating Fields for Glioblastoma Therapy During the COVID-19 Pandemic. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.679702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L., Zhou K., Chen J. Letter: The Impact of COVID-19 on the Neurosurgery Department During and After the Lockdown of Wuhan. Neurosurg Open. 2021;2(1):okaa020. doi: 10.1093/neuopn/okaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hafazalla K., Sahgal A., Jaja B., Perry J.R., Das S. Procarbazine, CCNU and vincristine (PCV) versus temozolomide chemotherapy for patients with low-grade glioma: a systematic review. Oncotarget. 2018;9(72):33623–33633. doi: 10.18632/oncotarget.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borrelli M., Corcione A., Castellano F., Fiori Nastro F., Santamaria F. Coronavirus Disease 2019 in Children. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.668484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ciuca I.M. COVID-19 in Children: An Ample Review. Risk Manag Healthc Policy. 2020;13:661–669. doi: 10.2147/RMHP.S257180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Capozza M.A., Triarico S., Attina G., Romano A., Mastrangelo S., Maurizi P., et al. Managing children with brain tumors during the COVID-19 era: Don't stop the care! Comput Struct Biotechnol J. 2021;19:705–709. doi: 10.1016/j.csbj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Millen G.C., Arnold R., Cazier J.B., Curley H., Feltbower R.G., Gamble A., et al. Severity of COVID-19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer. 2021;124(4):754–759. doi: 10.1038/s41416-020-01181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cancer Australia - Australian Government. Children and young people with cancer and COVID-19. 2021.
- 110.Carai A., Locatelli F., Mastronuzzi A. Delayed referral of pediatric brain tumors during COVID-19 pandemic. Neuro Oncol. 2020;22(12):1884–1886. doi: 10.1093/neuonc/noaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahluwalia R., Rocque B.G., Shannon C.N., Blount J.P. The impact of imposed delay in elective pediatric neurosurgery: an informed hierarchy of need in the time of mass casualty crisis. Childs Nerv Syst. 2020;36(7):1347–1355. doi: 10.1007/s00381-020-04671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fisher A.P., Patronick J., Gerhardt C.A., Radonovich K., Salloum R., Wade S.L. Impact of COVID-19 on adolescent and emerging adult brain tumor survivors and their parents. Pediatr Blood Cancer. 2021;68(9) doi: 10.1002/pbc.29116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sawyer J.L., Mishna F., Bouffet E., Saini M., Zlotnik-Shaul R. Bridging the Gap: Exploring the Impact of Hospital Isolation on Peer Relationships Among Children and Adolescents with a Malignant Brain Tumor. Child Adolesc Soc Work J. 2021:1–15. doi: 10.1007/s10560-021-00764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Y. Impact of the COVID-19 Pandemic on Biomedical and Clinical Research. Matter. 2020;3(4):970–973. doi: 10.1016/j.matt.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mallari R.J., Avery M.B., Corlin A., Eisenberg A., Hammond T.C., Martin N.A., et al. Streamlining brain tumor surgery care during the COVID-19 pandemic: A case-control study. PLoS ONE. 2021;16(7) doi: 10.1371/journal.pone.0254958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amoo M., Horan J., Gilmartin B., Nolan D., Corr P., MacNally S., et al. The provision of neuro-oncology and glioma neurosurgery during the SARS-CoV-2 pandemic: a single national tertiary centre experience. Ir J Med Sci. 2021;190(3):905–911. doi: 10.1007/s11845-020-02429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doglietto F., Vezzoli M., Biroli A., Saraceno G., Zanin L., Pertichetti M., et al. Anxiety in neurosurgical patients undergoing nonurgent surgery during the COVID-19 pandemic. Neurosurg Focus. 2020;49(6):E19. doi: 10.3171/2020.9.FOCUS20681. [DOI] [PubMed] [Google Scholar]
- 118.Vissio E., Falco E.C., Collemi G., Borella F., Papotti M., Scarmozzino A., et al. Impact of COVID-19 lockdown measures on oncological surgical activity: Analysis of the surgical pathology caseload of a tertiary referral hospital in Northwestern Italy. J Surg Oncol. 2021;123(1):24–31. doi: 10.1002/jso.26256. [DOI] [PubMed] [Google Scholar]
- 119.Krenzlin H., Bettag C., Rohde V., Ringel F., Keric N. Involuntary ambulatory triage during the COVID-19 pandemic - A neurosurgical perspective. PLoS ONE. 2020;15(6) doi: 10.1371/journal.pone.0234956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh R., Sahu A., Singh K., Prasad R.S., Pandey N., Singh R.C. Impact of COVID-19 Pandemic on Neurosurgical Practice in a Tertiary Care Center in India. J Neurosci Rural Pract. 2021;12(1):24–32. doi: 10.1055/s-0040-1716455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Samper Ots P.M., Zapatero Ortuno J., Pedraza Fernandez S., Mayrata Canellas E., Gonzalez San Segundo C., Campo Vargas M., et al. Impact of covid-19 on patients in radiotherapy oncology departaments in Spain. Radiother Oncol. 2021;161:148–151. doi: 10.1016/j.radonc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dyson E.W., Craven C.L., Tisdall M.M., James G.A. The impact of social distancing on pediatric neurosurgical emergency referrals during the COVID-19 pandemic: a prospective observational cohort study. Childs Nerv Syst. 2020;36(9):1821–1823. doi: 10.1007/s00381-020-04783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]