Fig. 4.
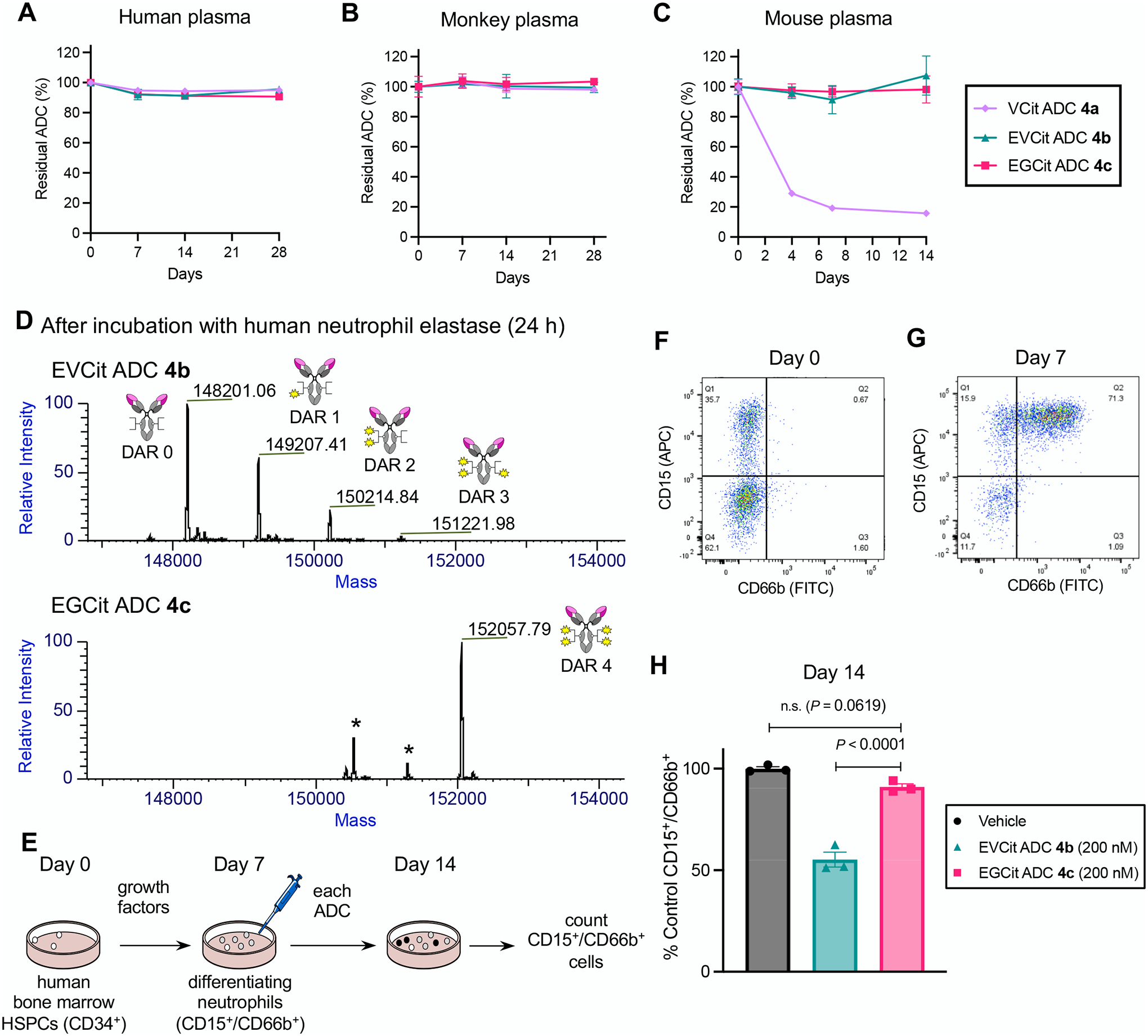
EGCit ADC is stable in plasma and spares differentiating human neutrophils derived from the bone marrow. A–C Stability at 37 °C in undiluted human plasma (A), cynomolgus monkey plasma (B), and BALB/c mouse plasma (C). VCit ADC 4a (light purple diamond), EVCit ADC 4b (green triangle), and EGCit ADC 4c (magenta square) were tested. D ESI-MS traces of ADCs 4b,c after incubation with human neutrophil elastase at 37 °C for 24 h. EGCit ADC 4c did not undergo cleavage, whereas EVCit ADC 4b underwent linker degradation and lost part of its payloads. E Study schedule for differentiation of human bone marrow HSPCs into neutrophils and subsequent treatment with ADCs 4b,c. After 3-day expansion (Day 0), HSPCs were treated with growth factors for 7 days and differentiated into CD15+ and CD66b+ granulocytes/neutrophils. F,G Flow cytometry before (Day 0, F) and after (Day 7, G) differentiation. H The effects of ADCs (vehicle, dark gray; EVCit ADC 4b, green; EGCit ADC 4c, magenta) on the population of human neutrophils relative to those of vehicle control (n = 3). All assays were performed in triplicate. Data are presented as mean values ± SEM. For statistical analysis, a one-way ANOVA with a Dunnett’s post hoc test was used (comparison control: EGCit ADC 4c). HSPCs, hematopoietic stem and progenitor cells.
