Abstract
This comprehensive systematic review and meta-analysis assessed the prevalence of Achilles tendinopathy (AT) in physical exercise (PE). Specifically, we estimate the overall risk of AT in physical exercise and compare sport-specific estimates of AT risk. PubMed, Web of Science, Cochrane Library, and SPORTDiscus were searched before the 1st of October 2021. Random-effects, subgroup analysis, sensitivity analysis and meta-regressions were conducted, involving 16 publications. This meta-analysis found that the overall prevalence of AT was 0.06 (95%CI, 0.04–0.07). The prevalence of Achilles tendon rupture was 0.03 (95%CI, 0.02–0.05). Subgroup analysis showed that the prevalence of AT increased with age, the highest among the group aged over 45 (0.08; 95%CI, 0.04–0.11), and the lowest among the group under 18 years old (0.02; 95%CI, 0.01–0.03). The gymnastics and ball games had the highest prevalence of AT, at (0.17; 95%CI, 0.14–0.20) and (0.06; 95%CI, 0.02–0.11), respectively. The prevalence of AT in athletes (0.06; 95%CI, 0.04–0.08) was higher than that of amateur exercisers (0.04; 95%CI, 0.02–0.06) and there was no difference in the prevalence of AT between males and females. There are differences in the prevalence of AT in different ages, sport events and characteristics of participants. This systematic review and meta-analysis suggested that it was necessary to pay more attention to AT in people who were older or engaged in gymnastics.
Keywords: Achilles tendinopathy, Prevalence, Physical exercise, meta-Analysis
Abbreviation list
- AT
Achilles tendinopathy
- CI
confidence interval
- NCAA
National Collegiate Athletic Association
- PE
physical exercise
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Introduction
Achilles tendinopathy (AT) is one of the most common injuries in physical exercise (PE), especially happens to athletes who are always engaged in long-term, continuous and high-intensity competition or training.1 AT is a degenerative disease,2 including tendinitis, accessory tendinitis, insertion tendinitis, retrocalcaneal bursitis and rupture,3 which is caused by the interaction of internal factors (e.g., lateral ankle instability, obesity, lower limb strength) and external factors (e.g., overuse, wrong training method, unsuitable sports equipment).4 The early symptoms of AT are mainly pain at the beginning or end of the exercise, and without movement at an advanced stage, which greatly affects the daily life of patients.5 AT is difficult to treat at present. Even after surgical treatment, AT may still occur repeatedly6 and the recurrence rate is as high as 44%.4 Nevertheless the huge harm of AT in exercise ability, it is not easy to be diagnosed in the early stage of disease because of its long incubation period.7 Therefore, AT has been easily underestimated and mishandled. Athletes with Achilles tendon rupture usually required a long period of treatment and recovery, which can also have a negative psychological and physiological impact on athletes.8, 9, 10
In recent years, the prevalence of AT has increased due to the increase in the number of people participating in PE.11 A large prospective cohort study found that the cumulative lifetime incidence of AT in male elite runners was more than 50%.12 Ames et al.13 reported that the risk of AT in runners was ten times higher than that in their inactive peers. A retrospective epidemiological study found that the incidence rate of AT in basketball athletes was 7.7%.14 In addition, an 11-year prospective follow-up study found that the incidence of AT in male professional football players reached 11.6%.15 Achilles tendon rupture has a devastating impact on Athletes’ athletic ability. A cohort study showed that more than 30% of professional athletes could not return to the field after Achilles tendon rupture, and even when players do return to the game, their appearance time and competition data were significantly worse than before.16 Moreover, AT also affected non-professional athletes, overweight people and inactive people, who suffered from loss of substantial working hours, and indirectly reduced economic income and quality of life.17,18
Systematic reviews on AT have mainly reviewed the treatment and prevention of AT.2,4,8,19,20 In addition, there was only one systematic review and meta-analysis21 on the prevalence of tendinopathy in children and adolescents. However, the meta-analysis on the prevalence of AT in PE has not been published. In view of this, we conducted a systematic review and meta-analysis of currently available studies which reported the prevalence of AT in PE. The primary aim of this meta-analysis is to estimate the overall risk of AT when participating in PE, and to compare estimated risks of AT for different ages, genders, sport events, characteristics of subjects, training years, and regions.
Materials and methods
Literature search
This meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.22 The relevant literature in each database that included PubMed, Web of Science, Cochrane Library, and SPORTDiscus was searched before October 1, 2021. The following search strategy was used: (“Exercise” OR “Physical Exercise” OR “Exercise Therapy”) AND (“Achilles tendinopathy” OR “Achilles tendon ruptures” OR “Achilles tendon injury”) AND (“Prevalence” OR “Period Prevalence” OR “Point Prevalence”) AND (“Cohort Study” OR “Incidence Study” OR “Cross Sectional Studies”), Specific search terms are shown in Supplementary Table 1.
Study selection
Two authors (YW and HZ) independently screened titles and abstracts, and then read through the full texts to check if the studies met the inclusion criteria. The third researcher (SC) arbitrated any discrepancies to reach a consensus. The inclusion criteria of this study were as follows: 1) physical exercise was performed by subjects; 2) the outcomes of concern were Achilles tendon injury or Achilles tendon disease or Achilles tendon rupture or Achilles tendinopathy. The exclusion criteria included 1) Achilles tendon injury was not caused by physical exercise; 2) the specific number or prevalence of AT was not mentioned; 3) there was no adequate data to get the target results.
Data extraction and quality assessment
Two experienced researchers (YW and HZ) independently carried out data extraction and quality evaluation. The extracted data included the first author's surname, publication year, study design, country, characteristics of subjects, participants' age, sport events, diagnostic criteria, number of patients with AT and total sample size. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)23 was used to evaluate the quality of the included studies.24
Statistical analysis
Considering differences in participants and types of exercise, we pooled estimates of the prevalence of AT using a random-effects meta-analysis. The Cochran Q-test of heterogeneity at a significance level of 5% was applied to evaluate statistical heterogeneity of the studies, and I2 was used for quantitative assessment of heterogeneity among the results according to the Higgins classification in which an I2 value above 75% was indicative of high heterogeneity.25 Subgroup analysis was conducted to find the potential source of heterogeneity according to age, gender, sport events, the characteristics of subjects and region. The source of heterogeneity was also tested by meta-regression analysis according to age and type of sports. In order to assess the robustness of the research results and evaluate the influence of each study on the overall prevalence, sensitivity analysis was performed via leave-one-out cross-validation,26 using I2 < 50% as the criterion. All analyses were performed using STATA version 11.0 (Stata Corp, College Station, TX, USA), with double data input to avoid input errors. The p < 0.05 was deemed as statistically significant unless specified elsewhere.
Results
Flow of study selection
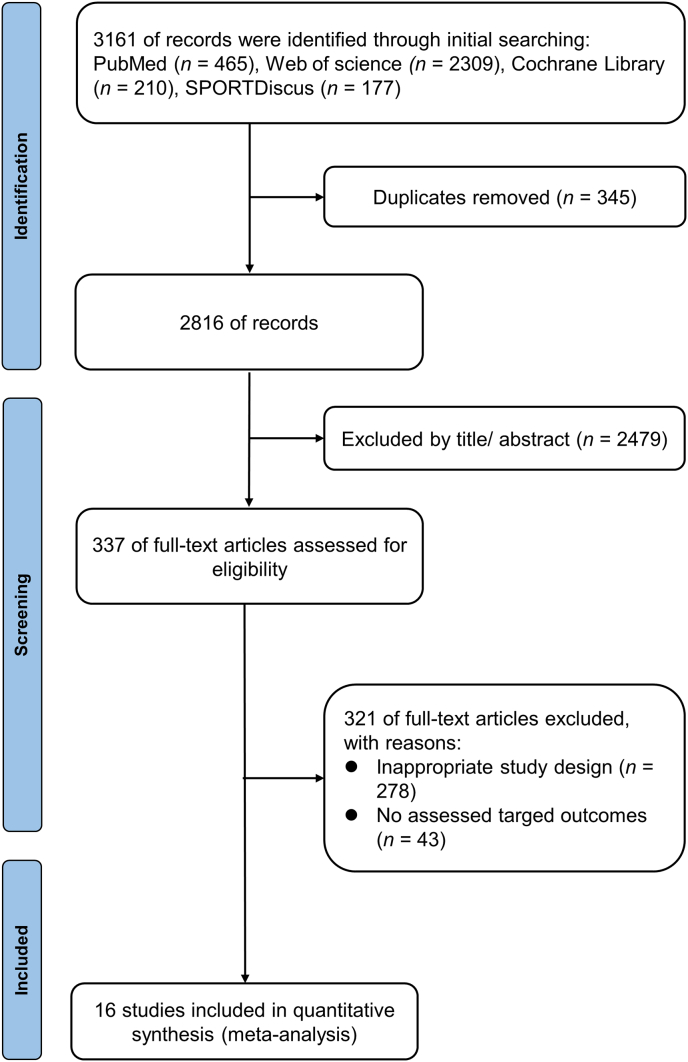
The flow of study selection was shown in Fig. 1. Initially, 3161 articles were retrieved and 345 duplicate articles were removed. 2479 articles were screened out by title and abstract, 278 articles were eliminated because of inappropriate design, and 43 articles were eliminated due to inconsistent outcome indicators. Therefore, 16 studies14,15,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 were eventually included in the final meta-analysis (see Table 1).
Fig. 1.
A flowchart showing the procedure used for study selection.
Table 1.
Characteristics of included studies in this meta-analysis (16 studies).
| Study | Year | Design | Country | Subject | Age (years) | Sport event | Diagnostic mode | AT/sample |
|---|---|---|---|---|---|---|---|---|
| Bonanno | 2021 | Cross section | America | current and former collegiate gymnasts, | / | Gymnastics | Information about Achilles tendon ruptures, gymnastics-related injuries, sport specialization, event/skills participation, and medication use were collected | 100/581 |
| Cassel | 2014 | Cohort | Germany | Adolescent athletes | 13.0 | Integrated Sports | Doppler ultrasound analysis | 14/760 |
| Cassel | 2018 | Cohort | Germany | Adolescent Elite athletes | 12.1 | Integrated Sports | Ultrasound examinations | 2/157 |
| Comin | 2012 | Cohort | Australia | Professional ballet dancers | 27.42 | Ballet | Ultrasound examinations | 3.5/79 |
| Emerson | 2010 | Cross section | England | Gymnasts | 16.3 | Gymnastics | Ultrasound examinations | 6/40 |
| Florit | 2019 | Cohort | Spain | Athletes | 26.4 | Integrated Sports | Team physicians diagnosed and classified tendinopathies according to anatomical location, sport types, playing category, sex, playing surface, lost training time, and severity (time to return to play) | 110/3839 |
| Ganse | 2014 | Cohort | Germany | European Veteran Athletics | 53.2 | Athletics | Report forms were used to identify injured athletes and injury types | 15/3154 |
| Gajhede-Knudsen | 2013 | Cohort | Sweden | Football players (man) | / | Football | Achilles tendon disorders were registered | 203/1743 |
| Hespanhol Junior | 2012 | Cross section | Brazil | Recreational runners | 43.0 | Running | Previous injuries history over the last 12 months | 8/200 |
| Hunt | 2016 | Cross section | America | College Athletes | / | Athletics | Evaluated the injury records of all varsity sports at a single NCAA Division 1 athletics program | 17/1076 |
| Lagas | 2019 | Cohort | Netherlands | Recreational runners | 41.9 | Running | The digital baseline questionnaire obtained at registration consisted of demographics, training characteristics, previous participation in events, lifestyle and previous running-related injuries | 100/1929 |
| Longo | 2009 | Cross section | Italy | European Veterans Athletics | 54.1 | Athletics | A fully trained orthopaedic surgeon made a diagnosis of Achilles tendinopathy according to clinicalcriteria | 85/178 |
| Nielsen | 2014 | Cohort | Denmark | Novice Runners | / | Running | All injured runners were diagnosed after a thorough clinical examination and then followed prospectively during their recovery | 18/933 |
| Ooi | 2015 | Cohort | Australia | Football athletes | 22.6 | Football | Using ultrasound and sonoelastography, 42 players were examined at baseline and again 9 months later (postseason) for the existence of intratendinous hypoechogenicity | 6/42 |
| Van Ginckel | 2009 | Cohort | Belgium | Novice runners | / | Running | AT was diagnosed as an insidious, gradual onset of mid-portion pain, palpated tenderness along the tendon, (morning) stiffness, tenderness and pain on exertion Structures in the differential diagnosis | 10/129 |
| Walls | 2010 | Cohort | Ireland | Professional Irish dancers | 25.8 | Dance | Magnetic resonance imaging | 14/18 |
Abbreviations: AT, Achilles tendinopathy; NCAA, National Collegiate Athletic Association.
Qualities of included studies
The detailed quality assessments are presented in Table 2. The quality score of all the included articles varied from 6 to 9. According to the STROBE scale, the quality of 6.25% studies were 10 scores, 12.5% studies were 9 scores, 50% studies were 8 scores, 18.75% studies were 7 scores and 12.5% studies were 6 scores (see Table 3).
Table 2.
Quality scores for assessing the risk of bias in the included articles.
| Study | Quality score |
|||||
|---|---|---|---|---|---|---|
| Sample population | Sample size | Participation | Outcome assessment | Analytical methods | Total score | |
| Bonanno, 2021 | 1 | 1 | 1 | 1 | 2 | 6 |
| Cassel, 2014 | 1 | 1 | 2 | 2 | 2 | 8 |
| Cassel, 2018 | 2 | 1 | 2 | 2 | 2 | 9 |
| Comin, 2012 | 1 | 1 | 2 | 2 | 2 | 8 |
| Emerson, 2010 | 1 | 2 | 2 | 2 | 2 | 9 |
| Florit, 2019 | 1 | 1 | 2 | 2 | 2 | 8 |
| Gajhede-Knudsen, 2013 | 1 | 1 | 2 | 2 | 2 | 8 |
| Ganse, 2014 | 1 | 1 | 2 | 2 | 2 | 8 |
| Hespanhol Junior, 2012 | 1 | 1 | 2 | 1 | 2 | 7 |
| Hunt, 2016 | 2 | 2 | 2 | 2 | 2 | 10 |
| Lagas, 2019 | 1 | 1 | 1 | 1 | 2 | 6 |
| Longo, 2009 | 1 | 1 | 2 | 1 | 2 | 7 |
| Nielsen, 2014 | 1 | 1 | 2 | 1 | 2 | 7 |
| Ooi, 2015 | 1 | 1 | 2 | 2 | 2 | 8 |
| Van Ginckel, 2009 | 1 | 1 | 2 | 2 | 2 | 8 |
| Walls, 2010 | 1 | 1 | 2 | 2 | 2 | 8 |
Table 3.
Results of subgroup analysis and publication bias stratified by study characteristics.
| Variables | Trials (n) | Prevalence (95%CI) | Heterogeneity |
|
|---|---|---|---|---|
| I2 (%) | p2 | |||
| Overall | ||||
| Achilles tendinopathy | 23 | 0.06 (0.04–0.07) | 96.8 | <0.001 |
| Achilles tendon rupture | 3 | 0.03(0.02–0.05) | 98.2 | <0.001 |
| Age | ||||
| <18 | 5 | 0.02 (0.00–0.03) | 37.2 | 0.173 |
| 18–45 | 9 | 0.05 (0.03–0.07) | 91.5 | <0.001 |
| ≥45 | 3 | 0.08 (0.04–0.11) | 98.5 | <0.001 |
| Gender | ||||
| Men | 9 | 0.05 (0.03–0.07) | 97.7 | <0.001 |
| Women | 8 | 0.05 (0.03–0.08) | 95.6 | <0.001 |
| Mixed | 6 | 0.09 (0.05–0.14) | 96.7 | <0.001 |
| Sport events | ||||
| Athletics | 5 | 0.04 (0.02–0.06) | 97.7 | <0.001 |
| Gymnastics | 3 | 0.17 (0.14–0.20) | 0 | 0.822 |
| Balls | 4 | 0.06 (0.02–0.11) | 97.7 | <0.001 |
| Run | 5 | 0.04 (0.02–0.06) | 87.1 | <0.001 |
| Others | 6 | 0.06 (0.02–0.11) | 92.1 | <0.001 |
| Characteristics | ||||
| Athletes | 18 | 0.06 (0.04–0.08) | 97.2 | <0.001 |
| Amateur exercisers | 5 | 0.04 (0.02–0.06) | 87.1 | <0.001 |
| Training years | ||||
| <5 | 4 | 0.02 (0.00–0.04) | 51.7 | 0.102 |
| ≥5 | 2 | 0.08 (0.00–0.15) | 95.7 | <0.001 |
| Region | ||||
| Oceania | 2 | 0.08 (−0.01 to 0.18) | 64.5 | 0.093 |
| Europe | 17 | 0.06 (0.04–0.07) | 97.2 | <0.001 |
| America | 4 | 0.06 (0.01–0.10) | 96.9 | <0.001 |
CI, confidence interval; p1value for prevalence; p2 value for heterogeneity in the subgroup; significant p-values are highlighted in bold prints.
Characteristics of included studies
The final sample consisted of 14858 participants, with the mean age ranging from 12.1 to 54.1 years. All included articles were published from 2009 to 2021, nine of which were published after 2014 years, 11 of which were cohort studies. Different events of sports were involved, including track and field, running, gymnastics, ball games and dance. Moreover, 11 of the 16 articles (68.75%) were conducted in the region of the European, 2 of the 16 articles (11.80%) were conducted in the Oceania region and 3 of the 16 articles (17.60%) were conducted in the American region.
Pooled prevalence of Achilles tendinopathy
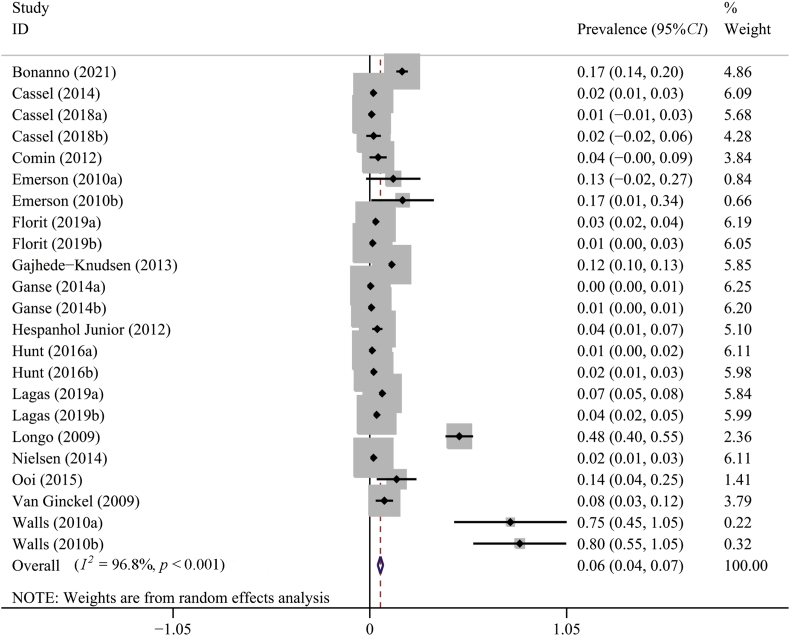
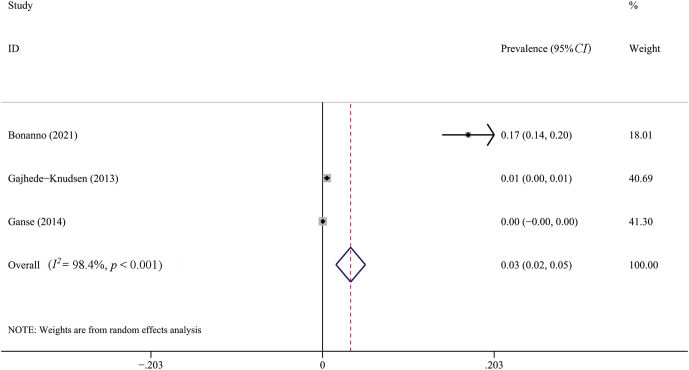
The results of overall meta-analyses were given in Table 2. The meta-analysis using the random-effect model found that the overall prevalence of AT was 0.06 (95%CI, 0.04–0.07). According to the results of I2 and χ2 test, there was a high degree of heterogeneity in the primary meta-analysis (I2 = 96.8%; p < 0.001) (Fig. 2). The prevalence of Achilles tendon rupture was 0.03 (95%CI, 0.02–0.05) with a high heterogeneity between studies, as shown in Fig. 3.
Fig. 2.
Forest plot of the pooled prevalence of Achilles tendinopathy from 16 studies (random effects model). ID, identity; CI, confidence interval.
Fig. 3.
Forest plot of the pooled prevalence of Achilles tendon rupture from 3 studies (random effects model). ID, identity; CI, confidence interval.
Stratified prevalence of Achilles tendinopathy
Subgroup analysis was conducted according to the subjects’ age, gender, sport events, characteristics and region. The results of subgroup analyses were given in Table 2. From the age point of view, the prevalence of AT increased with age. As shown in Fig. 4A, the prevalence of AT under the age of 18 was 0.02 (95%CI, 0.00–0.03), the prevalence of AT from 18 to 45 was 0.05 (95%CI, 0.03–0.07), and the prevalence of AT over the age of 45 was 0.08 (95%CI, 0.04–0.11). In terms of gender, there was no difference in prevalence between males and females (Fig. 4B). From the perspective of sports events, the two sports with the highest prevalence of AT were gymnastics and ball games, at (0.17; 95%CI, 0.14–0.20) and (0.06; 95%CI, 0.02–0.11), respectively. The lowest prevalence occurred in athletics and running, both were 0.04 (95%CI, 0.02–0.06) (Fig. 4C). From the perspective of characteristics of subjects, the prevalence of AT in athletes (0.06; 95%CI, 0.04–0.08) was higher than that of amateur exercisers (0.04; 95%CI, 0.02–0.06) (Fig. 4D). In terms of training years, the prevalence of AT with training years less than 5 years (0.02; 95%CI, 0.00–0.04) was far lower than those with training years more than 5 years (0.08; 95%CI, 0.00–0.15). Looking at the region distribution, the prevalence in Oceania (0.08; 95%CI, −0.01-0.18) was higher than that in Europe (0.06; 95%CI, 0.04–0.07) and America (0.06; 95%CI, 0.01–0.10).
Fig. 4.
The subgroup analysis of the prevalence of Achilles tendinopathy stratified by age (A), gender (B), sport events (C) and characteristics (D). ID, identity; CI, confidence interval.
Meta-regression analysis
Two variables (age and sport events) were first assessed in univariable meta-regression analyses, so as to find the source of heterogeneity. The results of univariable meta-regression analyses demonstrated that sport events and age were not correlated with the prevalence of AT.
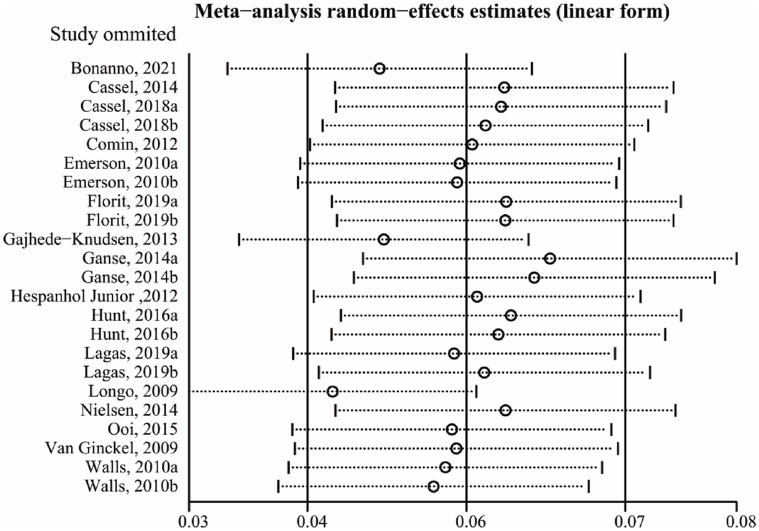
Sensitivity analysis
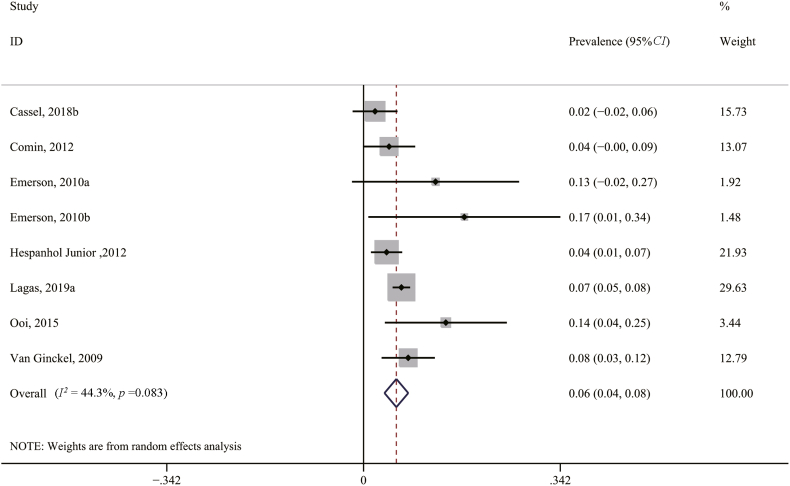
In order to further examine the source of heterogeneities and the robustness of the results, we performed leave-one-out cross-validation for sensitivity analysis. As shown in Fig. 5, the results of leave-one-out cross-validation suggested that 9 studies mainly contributed to the heterogeneity in the primary meta-analysis (I2 = 52.5%). After excluding 9 studies, the prevalence of AT was 0.06 (95%CI, 0.04–0.08, p = 0.083, I2 = 44.3%) (Fig. 6).
Fig. 5.
Sensitivity analysis of the prevalence of Achilles tendinopathy by leave-one-out cross validation.
Fig. 6.
Forest plot of the pooled prevalence of Achilles tendinopathy after excluding 9 studies. ID, identity; CI, confidence interval.
Discussion
This is the first meta-analysis to estimate the pooled prevalence of AT in PE, based on 16 observational studies. Our study mainly found that the total prevalence of AT in PE was 6%, and the prevalence of Achilles tendon rupture was 3%. Subgroup analysis showed that the top two sport events with the highest prevalence of AT were gymnastics and ball games, the prevalence of AT was the highest in people aged over 45 years, reaching 8%, and the lowest in people younger than 18, only 2%. The prevalence of AT of athletes was higher than that of amateur exercisers. The prevalence of AT in Oceania is higher than that in Europe and America. There was no significant difference in the prevalence of AT between genders.
Our result showed that the incidence of Achilles tendon rupture in PE was 3%, but only three articles mentioned the number of Achilles tendon rupture in three sports, including athletics, football, and gymnastics, and the number of Achilles tendon rupture reported in gymnastics was much higher than that in athletics and football, which might increase the overall prevalence of Achilles tendon rupture. Our results showed that the prevalence of AT in people was increased with age. At present, the specific relationship between the prevalence of AT and age remains unclear, and AT did exist at any age.1 Our results were consistent with a previous systematic review and meta-analysis which showed that AT existed in adolescents, and the prevalence of AT would increase with age.41 At present, the prevalence of Achilles tendon rupture in PE is not clear, a study showed that the incidence of Achilles tendon rupture was higher in sedentary men aged 29–48.42 It was speculated that the relationship between AT and age may be due to the changes of mechanical properties of Achilles tendon with age.43 Recently, Sprague et al.44 found that there was a positive correlation between age and viscosity of uninjured Achilles tendon. Aging was usually associated with reduced cellular structure, increased glycoamine polysaccharide content, and decreased fibrous tissue,45,46 all of which can lead to degenerative changes in the Achilles tendon. At the same time, the aging tendon loses elasticity, forcing the muscle to work harder, eventually leading to changes in tendon structure.
Our present meta-analysis found that there was no significant difference in the incidence of AT between genders. Contrary to our findings, previous reviews have shown significant gender differences in AT prevalence, with higher rates in men than in women.41,47,48 Although inconclusive, most current findings support a higher susceptibility of men to AT.49, 50, 51 However, the common flaw in these findings is that the impact factors such as physical activity and weight are not standardized, which may confuse the real impact of gender.
Consistent with the previous study, we found that the prevalence of AT in gymnastics was significantly higher than in other sports.52 This may be related to the high training intensity of gymnasts and the heavy load on Achilles tendon. Some reviews showed that the prevalence of Achilles tendon injury in running and track and field sports is the highest. However, our results showed that the prevalence of AT in track and field and running is the lowest. Compared with the previous review, we found that the samples included in gymnastics were elite athletes, and an epidemiological study found that the incidence of AT in gymnasts was much higher than that in basketball athletes.49 Subgroup analysis showed that the prevalence of AT in ball games was 6%. Ball games generally require athletes to make a rapid judgment according to the competition form in a short time, such as repeated bounce, dribble pass, back and forth quick start and other technical actions with a large load on the Achilles tendon. These technical actions need the support of the Achilles tendon, which also lays a huge hidden danger for its injury.53,54
Our study found that athletes had a higher prevalence of AT because they had to play a lot of games each season, and even during the off-season, they still needed the training to maintain their competitive levels.14 Furthermore, AT in some professional athletes was asymptomatic and might be inaccurate in clinical diagnosis.20,52 And it took an average of more than 7.5 months for a professional player to recover from a torn Achilles tendon before returning to play for the first time.55 Our results showed that the prevalence of AT in Oceania was higher than that in European and American countries. The specific reason was not clear. It may be related to the exercise habits and economic development level of different countries.
This study also has many limitations. First, significant heterogeneity was found in the overall assessment of the prevalence of AT from 16 included studies. This heterogeneity was consistent with our expectations. The differences in duration and intensity of various sports and individual characteristics of participants might lead to the high heterogeneity between studies. Secondly, age could be regarded as an important source of heterogeneity, and only 3 included studies were older than 45 years old, which might reduce the credibility of the results of subgroup analysis. In addition, only three articles reported the number of Achilles tendon ruptures, thus the relationship between age and Achilles tendon rupture could not be further analyzed. Only four articles described the frequency of PE, and the statistical units were inconsistent. Finally, although the sample size included in the study was considerable, most articles did not mention the weekly training frequency and training details of athletes, and the number of studies available for each sport was limited, thus we could not fully explore the source of heterogeneity in this meta-analysis.
Conclusions
In conclusion, this systematic review and meta-analysis provide valuable insights into the prevalence of Achilles tendinopathy during exercise, which can be used as a reference risk for AT in specific sports at present, and can be used to compare the prevalence of AT in sports of different populations and types of sports. Our study suggests that it is necessary to pay more attention on AT in the people who are older or engaged in gymnastics.
Authors’ contributions
YW and SC participated in the study design, data analysis and drafted and critically revised the manuscript. HZ and SC were responsible for selecting articles for inclusion and conducted the risk of bias assessment. YW and ZN were responsible for data extraction and helped to revise the manuscript. All authors read and approved the manuscript.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smhs.2022.03.003.
Contributor Information
Yahai Wang, Email: 1158990200@qq.com.
Sidong Cui, Email: 1097048209@qq.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cardoso T.B., Pizzari T., Kinsella R., et al. Current trends in tendinopathy management. Best Pract Res Clin Rheumatol. 2019;33(1):122–140. doi: 10.1016/j.berh.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Magnan B., Bondi M., Pierantoni S., et al. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20(3):154–159. doi: 10.1016/j.fas.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Weinfeld S.B. Achilles tendon disorders. Med Clin. 2014;98(2):331–338. doi: 10.1016/j.mcna.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Silbernagel K.G., Hanlon S., Sprague A. Current clinical concepts: conservative management of achilles tendinopathy. J Athl Train. 2020;55(5):438–447. doi: 10.4085/1062-6050-356-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffulli N., Longo U.G., Kadakia A., et al. Achilles tendinopathy. Foot Ankle Surg. 2020;26(3):240–249. doi: 10.1016/j.fas.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Aicale R., Oliviero A., Maffulli N. Management of Achilles and patellar tendinopathy: what we know, what we can do. J Foot Ankle Res. 2020;13(1):59. doi: 10.1186/s13047-020-00418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavone V., Vescio A., Mobilia G., et al. Conservative treatment of chronic achilles tendinopathy: a systematic review. J Funct Morphol Kinesiol. 2019;4(3):46. doi: 10.3390/jfmk4030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schepsis A.A., Jones H., Haas A.L. Achilles tendon disorders in athletes. Am J Sports Med. 2002;30(2):287–305. doi: 10.1177/03635465020300022501. [DOI] [PubMed] [Google Scholar]
- 9.Longo U.G., Petrillo S., Maffulli N., et al. Acute achilles tendon rupture in athletes. Foot Ankle Clin. 2013;18(2):319–338. doi: 10.1016/j.fcl.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Deangelis J.P., Wilson K.M., Cox C.L., et al. Achilles tendon rupture in athletes. J Surg Orthop Adv. 2009;18(3):115–121. [PubMed] [Google Scholar]
- 11.Longo U.G., Ronga M., Maffulli N. Achilles tendinopathy. Sports Med Arthrosc Rev. 2018;26(1):16–30. doi: 10.1097/jsa.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 12.Kujala U.M., Sarna S., Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15(3):133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 13.Ames P.R., Longo U.G., Denaro V., et al. Achilles tendon problems: not just an orthopaedic issue. Disabil Rehabil. 2008;30(20-22):1646–1650. doi: 10.1080/09638280701785882. [DOI] [PubMed] [Google Scholar]
- 14.Florit D., Pedret C., Casals M., et al. Incidence of tendinopathy in team sports in a multidisciplinary sports club over 8 seasons. J Sports Sci Med. 2019;18(4):780–788. [PMC free article] [PubMed] [Google Scholar]
- 15.Gajhede-Knudsen M., Ekstrand J., Magnusson H., et al. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47(12):763–768. doi: 10.1136/bjsports-2013-092271. [DOI] [PubMed] [Google Scholar]
- 16.Trofa D.P., Miller J.C., Jang E.S., et al. Professional athletes' return to play and performance after operative repair of an achilles tendon rupture. Am J Sports Med. 2017;45(12):2864–2871. doi: 10.1177/0363546517713001. [DOI] [PubMed] [Google Scholar]
- 17.Aström M. Partial rupture in chronic achilles tendinopathy. A retrospective analysis of 342 cases. Acta Orthop Scand. 1998;69(4):404–407. doi: 10.3109/17453679808999056. [DOI] [PubMed] [Google Scholar]
- 18.Martimbianco A.L.C., Ferreira R.E.S., Latorraca C.O.C., et al. Photobiomodulation with low-level laser therapy for treating Achilles tendinopathy: a systematic review and meta-analysis. Clin Rehabil. 2020;34(6):713–722. doi: 10.1177/0269215520912820. [DOI] [PubMed] [Google Scholar]
- 19.Wilson F., Walshe M., O'Dwyer T., et al. Exercise, orthoses and splinting for treating Achilles tendinopathy: a systematic review with meta-analysis. Br J Sports Med. 2018;52(24):1564–1574. doi: 10.1136/bjsports-2017-098913. [DOI] [PubMed] [Google Scholar]
- 20.Knapik J.J., Pope R. Achilles tendinopathy: pathophysiology, Epidemiology, diagnosis, treatment, prevention, and screening. J Spec Oper Med. 2020;20(1):125–140. doi: 10.55460/QXTX-A72P. [DOI] [PubMed] [Google Scholar]
- 21.Simpson M., Rio E., Cook J. At what age do children and adolescents develop lower limb tendon pathology or tendinopathy? A systematic review and meta-analysis. Sports Med. 2016;46(4):545–557. doi: 10.1007/s40279-015-0438-0. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/s0140-6736(07)61602-x. [DOI] [PubMed] [Google Scholar]
- 24.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serban C., Sahebkar A., Ursoniu S., et al. Effect of sour tea (Hibiscus sabdariffa L.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2015;33(6):1119–1127. doi: 10.1097/hjh.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 27.Bonanno J., Cheng J., Tilley D., et al. Factors associated with achilles tendon rupture in women’s collegiate gymnastics. Sport Health. 2022;14(3):358–368. doi: 10.1177/19417381211034510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassel M., Baur H., Hirschmüller A., et al. Prevalence of Achilles and patellar tendinopathy and their association to intratendinous changes in adolescent athletes. Scand J Med Sci Sports. 2015;25(3):e310–e318. doi: 10.1111/sms.12318. [DOI] [PubMed] [Google Scholar]
- 29.Cassel M., Risch L., Intziegianni K., et al. Incidence of achilles and patellar tendinopathy in adolescent elite athletes. Int J Sports Med. 2018;39(9):726–732. doi: 10.1055/a-0633-9098. [DOI] [PubMed] [Google Scholar]
- 30.Comin J., Cook J.L., Malliaras P., et al. The prevalence and clinical significance of sonographic tendon abnormalities in asymptomatic ballet dancers: a 24-month longitudinal study. Br J Sports Med. 2013;47(2):89–92. doi: 10.1136/bjsports-2012-091303. [DOI] [PubMed] [Google Scholar]
- 31.Emerson C., Morrissey D., Perry M., et al. Ultrasonographically detected changes in Achilles tendons and self reported symptoms in elite gymnasts compared with controls - an observational study. Man Ther. 2010;15(1):37–42. doi: 10.1016/j.math.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Ganse B., Degens H., Drey M., et al. Impact of age, performance and athletic event on injury rates in master athletics - first results from an ongoing prospective study. J Musculoskelet Neuronal Interact. 2014;14(2):148–154. [PubMed] [Google Scholar]
- 33.Hespanhol Junior L.C., Costa L.O., Carvalho A.C., et al. A description of training characteristics and its association with previous musculoskeletal injuries in recreational runners: a cross-sectional study. Rev Brasileira Fisioterapia. 2012;16(1):46–53. [PubMed] [Google Scholar]
- 34.Hunt K.J., Hurwit D., Robell K., et al. Incidence and Epidemiology of foot and ankle injuries in elite collegiate athletes. Am J Sports Med. 2017;45(2):426–433. doi: 10.1177/0363546516666815. [DOI] [PubMed] [Google Scholar]
- 35.Lagas I.F., Fokkema T., Verhaar J.A.N., et al. Incidence of Achilles tendinopathy and associated risk factors in recreational runners: a large prospective cohort study. J Sci Med Sport. 2020;23(5):448–452. doi: 10.1016/j.jsams.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Longo U.G., Rittweger J., Garau G., et al. No influence of age, gender, weight, height, and impact profile in achilles tendinopathy in masters track and field athletes. Am J Sports Med. 2009;37(7):1400–1405. doi: 10.1177/0363546509332250. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen R.O., Rønnow L., Rasmussen S., et al. A prospective study on time to recovery in 254 injured novice runners. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ooi C.C., Schneider M.E., Malliaras P., et al. Sonoelastography of the achilles tendon: prevalence and prognostic value among asymptomatic elite Australian rules football players. Clin J Sport Med. 2016;26(4):299–306. doi: 10.1097/jsm.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 39.Van Ginckel A., Thijs Y., Hesar N.G., et al. Intrinsic gait-related risk factors for Achilles tendinopathy in novice runners: a prospective study. Gait Posture. 2009;29(3):387–391. doi: 10.1016/j.gaitpost.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 40.Walls R.J., Brennan S.A., Hodnett P., et al. Overuse ankle injuries in professional Irish dancers. Foot Ankle Surg. 2010;16(1):45–49. doi: 10.1016/j.fas.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Simpson M., Rio E., Cook J. At what age do children and adolescents develop lower limb tendon pathology or tendinopathy? A systematic review and meta-analysis. Sports Med. 2016;46(4):545–557. doi: 10.1007/s40279-015-0438-0. [DOI] [PubMed] [Google Scholar]
- 42.Barrios-Cárdenas A.L., Lazo-Vera J.O. [Epidemiological, clinical and therapeutic characteristics of Achilles tendon rupture] Acta Ortop Mex. 2021;35(3):252–256. [PubMed] [Google Scholar]
- 43.Hubbard R.P., Soutas-Little R.W. Mechanical properties of human tendon and their age dependence. J Biomech Eng. 1984;106(2):144–150. doi: 10.1115/1.3138471. [DOI] [PubMed] [Google Scholar]
- 44.Sprague A.L., Awokuse D., Pohlig R.T., et al. Relationship between mechanical properties (shear modulus and viscosity), age, and sex in uninjured Achilles tendons. Transl Sports Med. 2020;3(4):321–327. doi: 10.1002/tsm2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vailas A.C., Pedrini V.A., Pedrini-Mille A., et al. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol. 1985;58(5):1572–1576. doi: 10.1152/jappl.1985.58.5.1572. [DOI] [PubMed] [Google Scholar]
- 46.Ippolito E., Natali P.G., Postacchini F., et al. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62(4):583–598. [PubMed] [Google Scholar]
- 47.Lantto I., Heikkinen J., Flinkkilä T., et al. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand J Med Sci Sports. 2015;25(1):e133–e138. doi: 10.1111/sms.12253. [DOI] [PubMed] [Google Scholar]
- 48.Hess G.W. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- 49.Chan J.J., Chen K.K., Sarker S., et al. Epidemiology of Achilles tendon injuries in collegiate level athletes in the United States. Int Orthop. 2020;44(3):585–594. doi: 10.1007/s00264-019-04471-2. [DOI] [PubMed] [Google Scholar]
- 50.Wezenbeek E., De Clercq D., Mahieu N., et al. Activity-induced increase in achilles tendon blood flow is age and sex dependent. Am J Sports Med. 2018;46(11):2678–2686. doi: 10.1177/0363546518786259. [DOI] [PubMed] [Google Scholar]
- 51.Macchi M., Spezia M., Elli S., et al. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: a systematic review of clinical studies. Clin Orthop Relat Res. 2020;478(8):1839–1847. doi: 10.1097/corr.0000000000001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emerson C., Morrissey D., Perry M., et al. Ultrasonographically detected changes in Achilles tendons and self reported symptoms in elite gymnasts compared with controls--an observational study. Man Ther. 2010;15(1):37–42. doi: 10.1016/j.math.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Cristi-Sánchez I., Danes-Daetz C., Neira A., et al. Patellar and achilles tendon stiffness in elite soccer players assessed using myotonometric measurements. Sport Health. 2019;11(2):157–162. doi: 10.1177/1941738118820517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amin N.H., McCullough K.C., Mills G.L., et al. The impact and functional outcomes of achilles tendon pathology in national basketball association players. Clin Res Foot Ankle. 2016;4(3):205. doi: 10.4172/2329-910x.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee K.T., Hyuk J., Kim S.J. Return to play after open calcaneoplasty for insertional achilles tendinopathy with haglund deformity in competitive professional athletes. Orthop J Sports Med. 2021;9(6) doi: 10.1177/23259671211009820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.