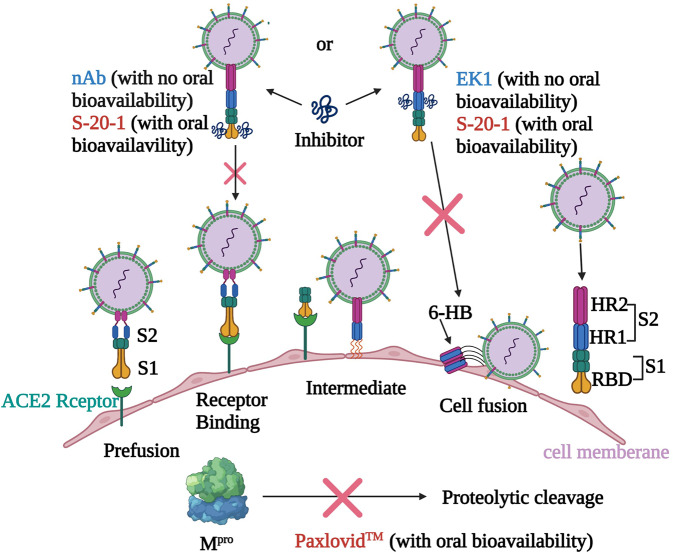
Fig. 10. Proposed mechanism of action of S-20-1 against SARS-CoV-2 infection.
The entry of SARS-CoV-2 into the host cell is initiated by binding of RBD in S1 subunit of S protein to ACE2 (the receptor of SARS-CoV-2), which triggers the conformation change of S2 subunit of S protein and exposes the fusion intermediate structure consisting of HR1, HR2, and fusion peptide (FP). Then, HR1 and HR2 interact with each other to form 6-HB, bringing the viral and host cell membranes together for fusion. Like SARS-CoV-2 nAb and EK1 peptide, S-20-1 is able to bind with RBD in S1 subunit and HR1 in S2 subunit to block viral attachment and fusion, respectively. Different from nAb and EK1 peptide, S-20-1 also has oral bioavailability like Paxlovid™, noted above, which targets the intracellular main protease (Mpro). However, S-20-1 is superior to peptide- and lipopeptide-based pan-CoV fusion inhibitors because it is much more resistant to proteolytic enzymes and has a longer half-life than EK1, as well as good oral bioavailability. Therefore, S-20-1 has better potential to be developed as an orally usable drug for treatment of SARS-CoV infection. The figure was created with BioRender.com.