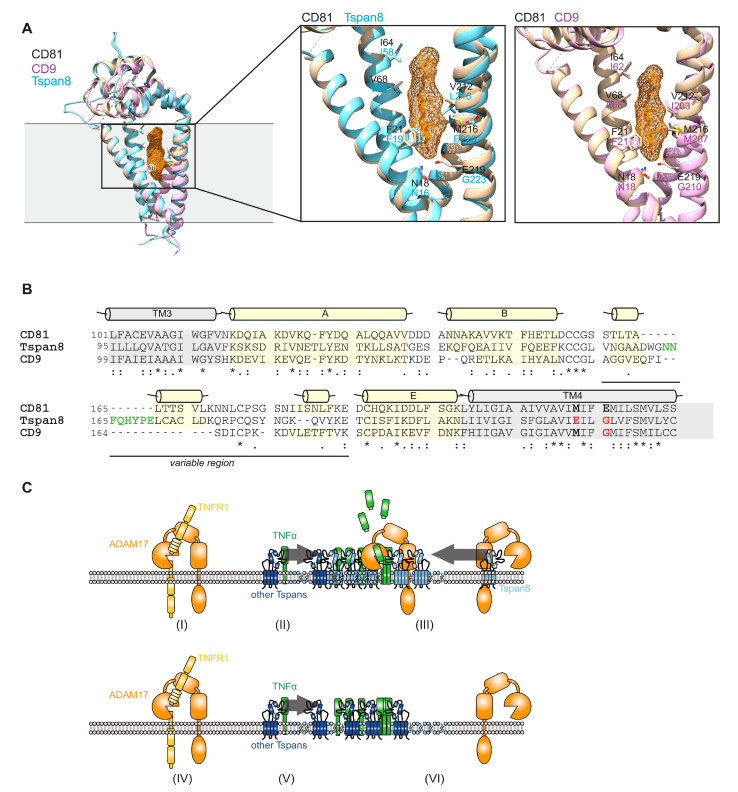
Figure 6.
Structural differences in the intramembrane potential cholesterol binding pocket might account for regulation of ADAM17 substrate availability. (A) Homology model of CD9 and Tspan8 superimposed onto the crystal structure of CD81 highlight structural differences in the intramembrane potential cholesterol binding pocket. (B) Sequence alignment of the large extracellular loop (LEL) region between transmembrane helix 3 (TM3) and transmembrane helix 4 (TM4) reveals differences in sequence and length within the variable region. Amino acid insertions in the Tspan8 LEL are highlighted in green. Variations in amino acids of TM4 contributing to the cholesterol binding pocket in CD81 are marked in red. * denotes amino acid conservation, indicates strong similarity, indicates weak similarity. (C) Proposed model for Tspan8-mediated substrate selectivity of ADAM17. (I+IV) Substrates that do not bind to Tspan8 are cleaved outside tetraspanin enriched microdomains (TEMs). (II) Tetraspanins other than Tspan8 recruit TNF α into TEMs. (III) Tspan8 recruits ADAM17 into TEMs, enabling ADAM17-mediated release of soluble TNF α. (V) In the absence of Tspan8, TNF α is still present in TEMs, while (VI) ADAM17 cannot be recruited, therefore reducing TNF α cleavage.