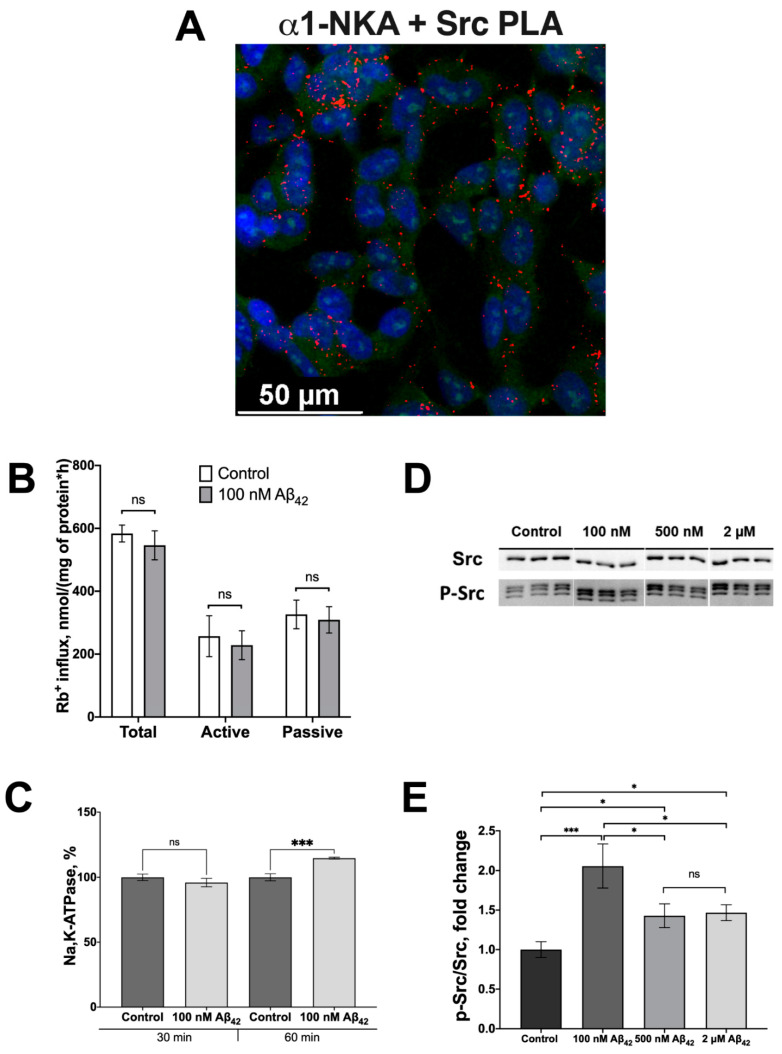
Figure 2.
Aβ42 activates Src-kinase in nanomolar concentrations. (A) Co-localization studies with Proximity Ligation Assay. Close proximity of Na,K-ATPase α1-subunit and Src kinase in the SH-SY5Y neuroblastoma cells. The confocal merged image of Hoechst fluorescence (blue), RNASelect (green) and Duolink Red (red) fluorescence is presented. Scale bar—50 µm. (B) The effect of Aβ42 on the Na,K-ATPase transport activity in SH-SY5Y cells. K+ (Rb+) influx after 30 min treatment with 100 nM Aβ42. Total Rb+ influx into the cells was measured in the absence of ouabain (Total); “Passive” denotes ouabain-resistant component of Rb+ influx in the sample where ouabain was added. Difference between the total and the passive fluxes gives the active (Active) Rb+ influx mediated by the Na,K-ATPase. (C) The changes in Na,K-ATPase levels in SH-SY5Y neuroblastoma cells after 30 or 60 min of incubation with 100 nM Aβ42. Na,K-ATPase levels were evaluated by flow cytometry. (D,E) Dose-dependent activation of Src by Aβ42. The ratio of phospho(Tyr)-416 Src to the total Src has been calculated. The phosphorylated and total Src levels have been measured with Western blot in SH-SY5Y neuroblastoma cells treated with 100 nM, 500 nM and 2 µM of Aβ42 for 30 min and normalized for control. Mean values ± SD from at least three independent experiments are shown. *—p < 0.05, ***—p < 0.001 compared to the control, ns—nonsignificant.