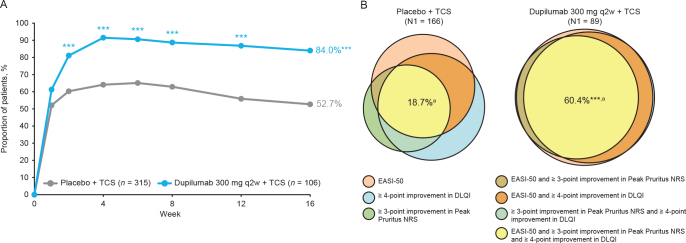
Fig. 1.
(A) Percentage of patients achieving Eczema Area and Severity Index (EASI)-50 or ≥ 3-point improvement in Peak Pruritus NRS score or ≥ 4-point improvement in Dermatology Life Quality Index (DLQI) from baseline at week 16. (B) Percentage of patients achieving EASI-50 and ≥ 3-point improvement in Peak Pruritus NRS score and ≥ 4-point improvement in DLQI from baseline at week 16. Venn diagrams generated using areas proportional to the number of patients achieving 1, 2, or 3 of the individual endpoints relative to the number of patients achieving ≥ 1 endpoints (N1; placebo n = 166, dupilumab q2w n = 89). aPercentages in the Venn diagrams are calculated over the population in the treatment arm. ***p < 0.0001 vs placebo + TCS. DLQI: Dermatology Life Quality Index; EASI-50: ≥ 50% improvement from baseline in Eczema Area and Severity Index; NRS: numerical rating scale; q2w: every 2 weeks; qw: weekly; TCS: topical corticosteroids.