Abstract
Carbapenem and other antibiotic resistance genes (ARGs) can be found in plasmids in Acinetobacter , but many plasmid types in this genus have not been well-characterized. Here we describe the distribution, diversity and evolutionary capacity of rep group 13 (GR13) plasmids that are found in Acinetobacter species from diverse environments. Our investigation was prompted by the discovery of two GR13 plasmids in A. baumannii isolated in an intensive care unit (ICU). The plasmids harbour distinct accessory genes: pDETAB5 contains bla NDM-1 and genes that confer resistance to four further antibiotic classes, while pDETAB13 carries putative alcohol tolerance determinants. Both plasmids contain multiple dif modules, which are flanked by pdif sites recognized by XerC/XerD tyrosine recombinases. The ARG-containing dif modules in pDETAB5 are almost identical to those found in pDETAB2, a GR34 plasmid from an unrelated A. baumannii isolated in the same ICU a month prior. Examination of a further 41 complete, publicly available plasmid sequences revealed that the GR13 pangenome consists of just four core but 1186 accessory genes, 123 in the shell and 1063 in the cloud, reflecting substantial capacity for diversification. The GR13 core genome includes genes for replication and partitioning, and for a putative tyrosine recombinase. Accessory segments encode proteins with diverse putative functions, including for metabolism, antibiotic/heavy metal/alcohol tolerance, restriction-modification, an anti-phage system and multiple toxin–antitoxin systems. The movement of dif modules and actions of insertion sequences play an important role in generating diversity in GR13 plasmids. Discrete GR13 plasmid lineages are internationally disseminated and found in multiple Acinetobacter species, which suggests they are important platforms for the accumulation, horizontal transmission and persistence of accessory genes in this genus.
Keywords: Acinetobacter, plasmids, Microbial Genomics, dif modules
Data Summary
Sequencing reads and the complete sequences of the chromosomes and plasmids of A. baumannii DETAB-E227 and A. baumannii DETAB-B39 are available from NBCI BioSample accessions SAMN18498586 and SAMN18498587, respectively, under BioProject accession PRJNA716893. Complete sequences can be found in the GenBank nucleotide database under accession numbers CP073060-CP073061 and CP072526-CP072529. Supplementary files are available on Figshare (https://doi.org/10.6084/m9.figshare.19494596) [1] .
Impact Statement.
Acinetobacter species are particularly well-adapted for persistence in hospital environments where they pose a life-threatening infection risk to the most clinically vulnerable patients. Plasmids with the potential to transfer multiple antibiotic resistance determinants between Acinetobacter species are therefore concerning, but most are not well-characterized. This work sheds further light on the poorly understood mobile gene pool associated with Acinetobacter . We show here that GR13 plasmids carry a small set of core genes but have access to a highly diverse set of accessory segments that might provide fitness advantages under certain conditions. The complex evolutionary dynamics of GR13 plasmids appear to be driven by the exchange of dif modules and by the actions of a diverse population of insertion sequences. The novel dif modules characterized here emphasize the broader importance of these elements to the dissemination of accessory genes in Acinetobacter . This study has improved our understanding of the diversity and distribution of dif modules, plasmids that carry them, and how both disseminate in the continuum of Acinetobacter populations that link hospitals and the wider environment.
Introduction
Acinetobacter is a genus of Gram-negative coccobacilli that are typically found in soils and moist environments but are also well-adapted for persistence in hospital settings [2]. A. baumannii is the most prominent pathogenic species and can cause human infections with high mortality rates, particularly given some strains exhibit extensive antibiotic resistance that severely compromises treatment [2, 3]. While less commonly reported, drug-resistant infections caused by other Acinetobacter species are an emerging threat [4–8].
Plasmids in Acinetobacter are typed according to replication initiation gene (rep) identity and have been divided into rep groups (GRs) [9]. A recent review listed 33 rep groups [10], and we have since described an additional group, GR34 [11]. Plasmids carrying clinically significant antibiotic resistance genes (ARGs) have been reported in A. baumannii [12–16] and in other Acinetobacter species [7, 8, 17–19], clearly indicating their important role in the emergence and transmission of antimicrobial resistance in this genus. Few plasmid groups have been the subject of comparative analyses, so how the remaining types evolve or are distributed, geographically and throughout the Acinetobacter genus, is poorly understood and their genetic structures remain largely undescribed.
Some Acinetobacter plasmids carry multiple pairs of recombination sites that resemble chromosomal dif sites, which are targets for XerC and XerD tyrosine recombinases [20]. These have been called plasmid-dif (pdif) sites [12], and have been shown to be recognized by A. baumannii XerC and XerD [21]. ARGs have been found in pdif-flanked structures called dif modules, including the carbapenemase genes bla OXA-24 [22], bla OXA-58 [23] and bla OXA-72 [24], the tetracycline resistance gene tet(39) [12], the macrolide resistance genes msr(E)-mph(E) [12], the aminoglycoside resistance gene aacC2d and the sulphonamide resistance gene sul2 [11]. Identical ARG-containing dif modules have been found in multiple contexts and in different types of plasmids [12]. Further dif modules, including those carrying genes for chromium resistance, a serine recombinase, RND efflux system and multiple toxin–antitoxin systems have also been described [11, 12, 25, 26]. Given the apparent importance of dif modules to the evolution of some Acinetobacter plasmids, it is important to understand the breadth of genetic cargo they carry and which types of plasmids can interact with them.
We recently described the GR34 family of plasmids that share a 10 kbp core segment but can grow to as large as 190 kbp through the acquisition of dif modules [11]. The exemplar GR34 plasmid, pDETAB2, is from an A. baumannii isolated in an intensive care unit (ICU) in Hangzhou, China, and carries six ARGs in a series of dif modules [11]. Here, we report two GR13-type plasmids found in unrelated A. baumannii isolated 1 and 2 months later in that same ICU, one cryptic and the other carrying ARG-containing dif modules identical to ones in pDETAB2. GR13 plasmids have not been studied in detail since the group was defined using A. baumannii plasmid p3ABAYE as a reference in 2010 [9]. In order to contextualize the differences between them, we undertook a detailed comparative analysis of the ICU GR13 plasmids and 41 complete GR13 plasmid sequences from GenBank. This facilitated the first evaluation of the distribution, gene content, structures and evolutionary characteristics of the GR13 plasmid family.
Methods
Ethics
Ethical approval and informed consent were obtained by the Sir Run Run Shaw Hospital local ethics committee (approval number 20190802–1).
Bacterial isolation and antibiotic susceptibility testing
DETAB-E227 was isolated from a cleaning cart surface swab and DETAB-P39 from a patient rectal swab in Sir Run Run Shaw Hospital Intensive Care Unit in Hangzhou, China in 2019. Both samples were cultured on CHROMagar (CHROMagar, Paris, France) containing 2 mg l−1 meropenem at 37 °C for 24 h. Isolated colonies of presumptive A. baumannii were sub-cultured on Mueller–Hinton agar (MHA) (Oxoid, Hampshire, UK) and incubated at 37 °C for 24 h. MICs for imipenem, meropenem, tobramycin, gentamicin, ciprofloxacin, levofloxacin, ceftazidime, colistin and tigecycline were determined using broth microdilution with results interpreted according to CLSI 2019 guidelines.
Plasmid transfer assays
DETAB-E227 was filter-mated with a rifampicin-resistant derivative of A. baumannii ATCC 17978 or A. nosocomialis strain XH1816 as described previously [27]. XH1816 is a colistin-resistant, meropenem-sensitive clinical A. nosocomialis strain that was isolated from a human urine sample in 2011. Transconjugants were selected on MHA supplemented with rifampicin (50 µg ml−1) and meropenem (8 µg ml−1). The identity of transconjugants was confirmed through PFGE fingerprinting after digestion of genomic DNA with ApaI. Transconjugants were tested for the presence of pDETAB4 and pDETAB5 by PCR with primers that target the replication genes of each plasmid (Table S1). ATCC 17978 transconjugants containing pDETAB2 were mated with XH1816 as above, with transconjugants selected on MHA supplemented with colistin (2 µg ml−1) and meropenem (8 µg ml−1).
S1 nuclease digestion, PFGE and Southern blot
To confirm transfer had occurred, plasmids were visualized following S1 nuclease treatment via PFGE, and the locations of resistance genes were confirmed via Southern blot as described previously [28]. Briefly, genomic DNA was digested with S1 nuclease (TaKaRa, Kusatsu, Japan) at 37 °C for 20 min. Treated DNA was loaded on a 1 % agarose Gold gel and PFGE was performed at 14 °C for 18 h, with 6 V/cm and pulse times from 2.16 to 63.8 s using the Bio-Rad CHEF-Mapper XA machine (Bio-Rad, CA, USA). DNA was transferred to a positively charged nylon membrane (Millipore, Billerica, MA, USA) by the capillary method and hybridized with digoxigenin-labelled bla OXA-58 and bla NDM-1-specific probes with an NBT/BCIP colour detection kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. XbaI-treated genomic DNA from Salmonella enterica H9812 was used as a size marker.
Whole-genome sequencing and analysis
Genomic DNA was extracted from A. baumannii DETAB-E227 and DETAB-P39 using a Qiagen minikit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Whole-genome sequencing was performed using both the Illumina HiSeq (Illumina, San Diego, USA) and the Oxford Nanopore GridION (Nanopore, Oxford, UK) platforms (Tianke, Zhejiang, China). Nanopore reads were trimmed with Filtlong v0.2.0 (https://github.com/rrwick/Filtlong) under default settings targeting approximately 100-fold genome coverage and Illumina sequence reads were trimmed with Shovill v1.1.0 under default (https://github.com/tseemann/shovill). De novo assembly of the Illumina and Nanopore reads was performed using Unicycler v0.4.8 [29]. MLST with the Pasteur and Oxford schemes was performed using mlst (https://github.com/tseemann/mlst) [30, 31].
Plasmid characterization
For alignment and visualization, all plasmids were opened in the same orientation and at the same position 48 bp upstream of the GR13 rep gene. ARGs and rep genes were identified using ABRicate v0.8.13 (https://github.com/tseemann/abricate) with the ResFinder [32] and pAci (File S1) databases, respectively. Insertion sequences were identified using the ISFinder database [33]. To screen the entire plasmid collection, an offline version of the ISFinder nucleotide database was constructed from an available version from October 2020 (https://github.com/thanhleviet/ISfinder-sequences). The database was used with abricate, initially with a minimum nucleotide identity threshold of 80 % and coverage threshold of 90 % to identify putative novel IS. Representative sequences with greater than 90 % coverage and between 80 and 95% nucleotide identity were validated manually and those that represented novel IS (Table S2) were submitted to ISFinder, named, and added to the database. The resulting database was used with 95 % identity and coverage thresholds, and sequences identified were considered isoforms of the representative IS or putative IS in accordance with ISFinder’s directions for isoform identification. Gene Construction Kit (Textco Biosoftware, Raleigh, USA) was used to annotate and examine plasmid sequences.
Plasmid pangenome analysis
Plasmids were annotated with Prokka 1.14.6 [34], using reference protein sequences to standardise annotations. Reference sequences were obtained from the NCBI Identical Protein Groups resource by querying ‘Acinetobacter[Organism] AND (uniprot[filter] OR refseq[filter])’. As insertion sequences were analysed separately, lines matching ‘transposase’ or ‘product=IS’ were removed from gff annotation files. Pangenomes and a core-gene alignment were constructed from these annotations using Panaroo 0.1.0 [35], reducing contamination-removal processes using --mode relaxed --no_clean_edges --min_trailing_support 0 min_edge_support_sv 0 --trailing_recursive 0 to reflect the use of complete sequences of highly mosaic plasmids. Functional annotation based on the eggNog orthology database version 5.0.2 [36] was performed with emapper-2.1.6–43-gd6e6cdf [37] using Diamond version 2.0.13 [38] for protein sequence alignments.
Core-gene analysis
Plasmid rep gene sequences were extracted manually, then aligned using mafft version 7 [39] with the GNS-i iterative refinement method and additional parameters, --reorder --anysymbol --maxiterate 2 --retree 1 –globalpair. Low-confidence residues in the alignment were masked with GUIDANCE2 [40]. Phylogenies were constructed from the rep gene alignment using RaxML version 8.2.12 [41] and the GTRGAMMA model with automated bootstrapping.
For investigation of core-gene recombination, blastn was used to query all plasmid sequences with parA, parB and tyr13 from reference plasmid p3ABAYE and identify their homologues. The resulting sequences were aligned with mafft as described above and a neighbour-joining phylogeny was constructed. BAPS was used to partition all core-gene phylogenies and the highest level of BAPS discrimination was used to define distinct core-gene variants.
Results
Carbapenem-resistant DETAB-E227 carries a multidrug resistance GR13-type plasmid
DETAB-E227 was resistant to imipenem, meropenem, ceftazidime, gentamicin, tobramycin and ciprofloxacin, but susceptible to colistin and tigecycline (Table S3). The complete genome of DETAB-E227 includes a 3 749 086 bp chromosome and three plasmids, pDETAB4, pDETAB5 and pDETAB6 (Table 1). DETAB-E227 is a novel sequence type according to the Pasteur (STIP1554: cpn60-3, fusA-3, gtlA-2, pyrG-79, recA-3, rplB-4, rpoB-4) and Oxford (STOX2210: cpn60-1, gdhB-208, gltA-1, gpi-171, gyrB-231, recA-1, rpoD-153) MLST schemes.
Table 1.
DETAB-E227 and DETAB-P39 genome characteristics
|
Isolate |
DNA element |
Replicon type |
Size (bp) |
% GC |
Antibiotic resistance genes |
|---|---|---|---|---|---|
|
DETAB-E227 |
chromosome |
– |
3 749 086 |
39.1 |
–* |
|
|
pDETAB4 |
GR24 |
113 682 |
42.0 |
sul2, tet(B) |
|
|
pDETAB5 |
GR13 |
97 035 |
41.5 |
bla OXA-58, bla NDM-1, ble MBL, sul2, aacC2d, msr(E)-mph(E) |
|
|
pDETAB6 |
Aci3 |
7145 |
32.0 |
– |
|
DETAB-P39 |
chromosome |
– |
3 877 093 |
38.9 |
–† |
|
pDETAB13 |
GR13 |
91 083 |
39.9 |
– |
*The DETAB-E227 chromosome contains the intrinsic genes oxaAb (OXA-24) and ampC (96.6 % identical to bla ADC-25).
†The DETAB-P39 chromosome contains the intrinsic genes oxaAb (OXA-88) and ampC (95.9 % identical to bla ADC-25).
Nine antibiotic resistance genes were found in the DETAB-E227 genome (Table 1). Two of these, bla ADC-25 and bla OXA-424, are the native ampC and oxaAb β-lactamase genes found in the chromosome. The sul2 and tet(B) genes are in the 113 682 bp GR24-type plasmid pDETAB4 and the remaining resistance genes, bla NDM-1, bla OXA-58, ble MBL, aacC2d [also called aac(3’)-IId], msr(E)-mph(E) and a second copy of sul2 are in the 97 035 bp GR13-type plasmid pDETAB5 (Table 1, Fig. 1a).
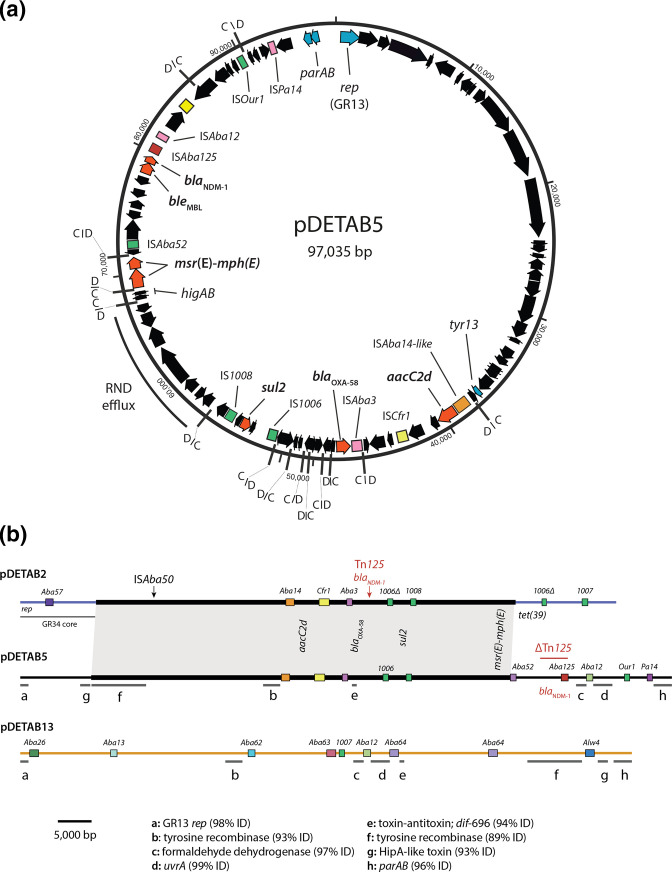
Fig. 1.
GR13 plasmid pDETAB5. (a) Circular map of pDETAB5 drawn from GenBank accession CP072528. Plasmid sequence is shown as a black line, with arrows inside representing ORFs. Coloured boxes represent IS. Black lines marked ‘C/D’ or ‘D/C’ represent pdif sites and indicate their orientations. Arrows filled with blue and labelled rep, parAB and tyr13 represent the GR13 genes of pDETAB5. (b) Linear maps of pDETAB2, pDETAB5 and pDETAB13, drawn to scale from GenBank accessions CP047975, CP072528 and CP073061. Near-identical sequences in pDETAB2 and pDETAB5 are bridged by grey shading and homologous regions of pDETAB5 and pDETAB13 are marked by lines labelled ‘a’ to ‘h’. IS are shown as coloured, labelled boxes and the locations of ARGs are indicated.
In three independent conjugation experiments, pDETAB5 transferred from DETAB-E227 to A. baumannii ATCC 17978 at a mean frequency of 6.96×10−7 transconjugants per donor (Table S4). The presence of pDETAB5 in ATCC 17978 transconjugants was confirmed using S1-PFGE, Southern blotting targeting the bla NDM-1 and bla OXA-58 genes, and PCR targeting the pDETAB5 rep gene (Fig. S1). pDETAB4 was not detected in transconjugants and pDETAB6 was not tested for. pDETAB5 did not transfer from DETAB-E227 to A. nosocomialis XH1816 or from ATCC 17978 to XH1816 in three independent experiments.
pDETAB5 resembles the GR34 plasmid pDETAB2
The combination of ARGs in pDETAB5 resembles that in the GR34 plasmid pDETAB2, found in a STIP138 A. baumannii isolated in the same ICU 1 month prior to DETAB-E227 [11]. pDETAB5 contains 14 pdif sites (Fig. 1a) and pDETAB2 contains 16. Alignment of pDETAB5 and pDETAB2 reveals that approximately 63 kbp of the pDETAB2 sequence is present in pDETAB5 (Fig. 1b). The sequence they share includes multiple dif modules but not the region of pDETAB2 that has been identified as core to GR34 plasmids [11]. The aacC2d, bla OXA-58 and msr(E)-mph(E)-containing dif modules in pDETAB2 and pDETAB5 are identical and their sul2-containing modules differ only through a 132 bp deletion in the copy of IS1006 in pDETAB2. Other dif modules shared by the plasmids encode HigAB-like and AbkAB-like toxin–antitoxins, a putative serine recombinase and a putative RND efflux system.
The bla NDM-1 and ble MBL genes in pDETAB5 and pDETAB2 are in different contexts. In pDETAB2, the bla NDM-1 and ble MBL genes are in a complete copy of Tn125 inserted in a 696 bp dif module that contains putative toxin–antitoxin genes [11]. This module is uninterrupted in pDETAB5 (Fig. 1a) and instead, the bla NDM-1 and ble MBL genes are in a partial copy of Tn125 that retains one copy of ISAba125 and 3062 bp of the passenger segment (labelled red line in Fig. 1b). This indicates that, despite sharing a collection of dif modules that must have a common origin, pDETAB2 and pDETAB5 acquired bla NDM-1 independently in distinct events.
pDETAB13 of carbapenem-sensitive DETAB-P39 is only distantly related to pDETAB5
The complete genome of DETAB-P39 includes a 3 877 093 bp chromosome and the 91 083 bp plasmid pDETAB13 (Fig. S2). DETAB-39 is STIP221 and STOX351. Despite growing on the initial meropenem-supplemented CHROMagar plate, DETAB-P39 was phenotypically sensitive to meropenem and to all other antibiotics tested (Table S3), and its genome does not contain any acquired antibiotic resistance genes.
The rep gene of pDETAB13 is 99.4 % identical to that of the reference GR13 plasmid pA3ABAYE and 98.3 % identical to that of pDETAB5. pDETAB13 contains nine pdif sites and nine complete insertion sequences (ISs), including the novel ISAba62, ISAba63 and ISAba64 (Fig. 1b and S2). Excluding ISs, just 20 998 bp of pDETAB13 is homologous to pDETAB5, but the shared sequences are split across eight regions that range from 99–93% identical (Fig. 1b). The shared segments include the rep gene and putative partitioning genes parAB in a contiguous region (a and h in Fig. 1b), and determinants for several proteins with putative functions: a HipA-like toxin, a toxin–antitoxin system, formaldehyde dehydrogenase, UvrA-like excision endonuclease, and two tyrosine recombinases (also called integrases, Pfam: PF00589). The toxin–antitoxin genes in pDETAB13 are found in a 696 bp dif module (dif-696b) that is 93.6 % identical to the one in pDETAB5 (dif-696a). Accumulated SNPs differentiate the dif-696 variants, suggesting that the presence of these modules in pDETAB5 and pDETAB13 is not the result of a recent horizontal transfer event.
Some notable ORFs in pDETAB13 are not shared by pDETAB5. A cluster of nine ORFs located in a 10 724 bp region, which we termed ADH, between ISAba62 and ISAba63 includes determinants for a putative transcriptional regulator, putative alcohol dehydrogenases and putative metabolic enzymes including a monooxygenase, amidotransferase, hydrolase, alkene reductase and oxidoreductase. A 2111 bp dif module, dif-2111, encodes a putative NAD(P)-dependent alcohol dehydrogenase and a LysR-family transcriptional regulator. Three other dif modules in pDETAB13 were not assigned functions as they encode hypothetical proteins.
GR13 plasmids have been collected from diverse sources
To characterize the GR13 plasmid family, we conducted a comparative analysis of publicly available sequences. The 1173 bp rep gene of reference GR13 plasmid p3ABAYE (GenBank accession CU459140) [9] was used to query the GenBank non-redundant nucleotide database (last search 9 August 2021), and 40 complete plasmid sequences containing rep genes that ranged from 99.1–97.3% identical to the query were found, along with a single plasmid with a rep gene 79.8 % identical to the query (Table S5). These GR13 plasmids are from A. baumannii , A. pittii , A. nosocomialis , A. johnsonii , A. soli, A. seifertii and A. radioresistans that were isolated from various cities in China as well as from Japan, Cambodia, Thailand, Vietnam, India, Pakistan, Australia, Chile, the USA, the Czech Republic, France, Germany and the Netherlands between 1986 and 2020 (Table S5). Sources of isolation ranged from human clinical specimens and hospital environments to terrestrial and marine animals and environments (Table S5). The plasmids range in size from 50 047 bp to 206 659 bp and three carry additional replication genes (Table S5), suggesting that they have formed cointegrates with plasmids from different rep groups.
The small GR13 core genome has been subject to recombination
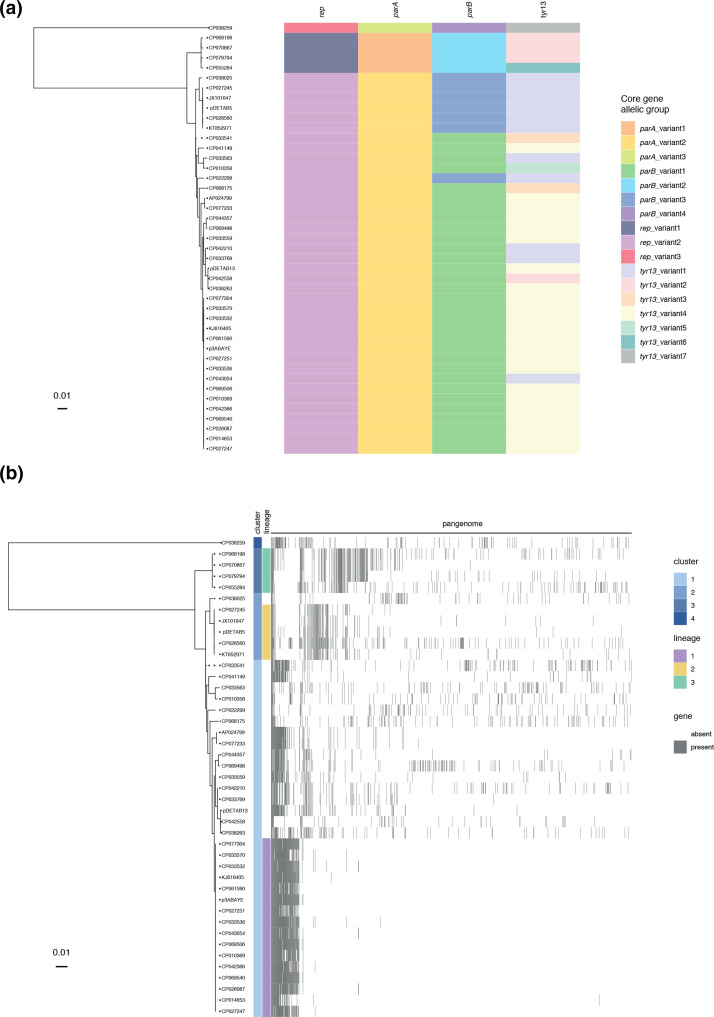
To characterize the gene content of GR13 plasmids, a pangenome was constructed. This consisted of 1190 genes: two considered core (present in 43 plasmids), two soft-core (42 plasmids), 123 shell (7 to 40 plasmids) and 1063 cloud (1 to 6 plasmids).
The four core genes were rep, the putative partitioning genes parA and parB, and a putative tyrosine recombinase gene that we will refer to as tyr13. Though parAB were found in only 42 of the 43 plasmids by the pangenome approach, using tblastn to query the remaining plasmid sequence (CP038259) with the amino acid sequences of ParA and ParB of pDETAB13 revealed equivalent genes with nucleotide identities of 78.8 and 79.8 %, respectively. The parAB genes were found adjacent to one another in all 43 plasmids and were usually adjacent to rep, but tyr13 was never found adjacent to rep or parAB.
The conservation of core genes was investigated by using BAPS to place gene sequences into allelic groups that differed by few SNPs and exhibited common SNP patterns that likely arose cumulatively from a recent ancestor. The distribution of parAB and tyr13 allelic groups were visualized relative to a rep gene phylogeny (Fig. 2a) and instances of recombination were identified where phylogenetic clusters did not contain parA, parB and tyr13 from the same allelic groups. Substitution of tyr13 genes appears to have occurred on multiple occasions while a single example of parB allele substitution was seen in CP022299.
Fig. 2.
The GR13 plasmid family pangenome. (a) Plasmid core-gene allelic group identities displayed relative to a rep gene phylogeny. (b) GR13 pangenome displayed relative to the rep gene phylogeny. Cluster and lineage memberships are indicated to the left of the pangenome visualization.
GR13 plasmid lineages have disseminated widely
The rep gene phylogeny was used to sub-type GR13 plasmids. The collection was partitioned into four broad-ancestry clusters of plasmids that, apart from CP022299, shared core replication and partitioning genes from the same allelic groups, reflecting their common ancestry. Further rep gene variation, as evident in the phylogenetic tree (Fig. 2b), indicated that clusters could be partitioned into epidemiologically relevant plasmid lineages. We have defined three GR13 lineages that are represented by four or more plasmids in this collection that are not separated by any SNPs in the rep gene phylogeny (Fig. S3), equivalent to a total rep identity of >99.8 % for lineage 1 (where the rep genes of three plasmids are missing a nucleotide in three different homopolymeric runs, likely due to sequencing errors) and 100 % for lineages 2 and 3. Plasmids in the same lineage share significant accessory gene content (Fig. 2b), consistent with them having descended from an ancestral plasmid that contained the same rep gene and a conserved set of accessory genes. The presence of accessory genes that differentiate some plasmids from other members of the same lineage highlight their capacity to diversify through gene acquisition.
Host species and sources of isolation varied within lineages, indicating that they have disseminated internationally and between Acinetobacter species. The best-represented lineage in this collection, lineage 1, contains the reference GR13 plasmid p3ABAYE and 15 others. p3ABAYE is from a clinical A. baumannii isolated in France in 2001, while other members of lineage 1 are from A. pittii , A. nosocomialis and A. seifertii strains from human clinical samples in various Chinese provinces, Australia, Colombia and Germany (Table S5). A single lineage 1 plasmid is derived from marine sediment. Lineage 1 has a well-conserved accessory genome, consisting of 39 core genes (present in all 16 plasmids), 58 shell genes (in two to 15 plasmids) and just two cloud genes (in one plasmid each). Lineage 2 plasmids include pDETAB5 and four other ARG-bearing plasmids. Representatives of lineage 2 have been found in A. baumannii , A. soli and an isolate of indeterminate Acinetobacter species derived from clinical samples, an ICU environment or sewage, but only in mainland China, Taiwan or Vietnam. In contrast, the four representatives of lineage 3 are from A. johnsonii or an indeterminate Acinetobacter that were isolated across wide geographic and temporal spans: soil from the USA in 1986, a spacecraft-associated clean room in the Netherlands in 2008, an intensive care unit sink in Pakistan in 2016 and bigeye tuna in China in 2018. Taken together, the distributions of lineage 1, 2 and 3 plasmids emphasize the capacity of GR13 plasmids for widespread dissemination and persistence.
Most accessory genes in GR13 plasmids are unique
There were 1090 gene families in the GR13 pangenome, with 4453 genes identified in total. Of the 1090 gene families, 745 could be assigned putative functions with our Prokka annotation and Panaroo approach (68.3 %), while 526 (48.2 %) and 442 (40.6 %) were assigned functions with the COG and KEGG schemes, respectively. COG placed gene families in broad functional categories, most commonly replication and repair (135/526, 25.7 %), transcription (83, 15.8 %) and inorganic transport and metabolism (78, 14.8 %) (File S2). KEGG categories offered more specific functional annotation and facilitated identification of the most common gene functions in the collection. Amongst the 50 most prevalent gene families that were assigned functions (File S2), families with putative metabolic functions were most common. The second most common gene families encode components of toxin–antitoxin systems, with HipA-like and Fic-like toxin-encoding genes the most abundant overall. Other functions of note included DNA integration and recombination (42 gene families, 194 genes, 43 plasmids), antimicrobial resistance (three gene families, six genes, five plasmids), heavy metal resistance (22 gene families, 51 genes, seven plasmids), alcohol tolerance (11 gene families, 172 genes, 32 plasmids), and phage defence (five gene families, 89 genes, 17 plasmids). Some functional groups contained multiple gene families, suggesting that genes with the same functions have been acquired on multiple occasions by different GR13 lineages.
Of the 1186 accessory genes, 746 (62.9 %) were found in just a single plasmid each. These so-called ‘singleton genes’ were found in 25 of the 43 plasmids, where they accounted for between 0.7 and 61.4 % of total gene content. The pangenome network showed that many singleton genes were found adjacent to one another in long, contiguous sequences that were unique to the plasmids that carried them (File S3). In CP028560 and CP069498, an abundance of singleton genes coincided with the presence of additional rep genes of types GR34 and GR24, respectively, indicating that plasmid cointegrate formation was associated with the introduction of significant numbers of genes.
ARG-bearing dif modules are subject to rearrangement by insertion sequences
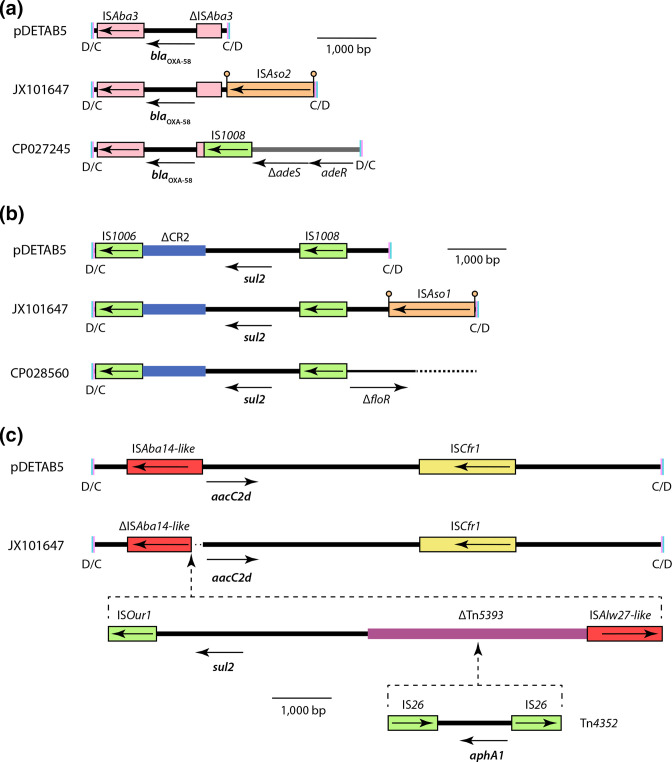
Lineage 2 plasmids and the only other ARG-containing plasmid, CP033563 from an A. nosocomialis isolated in Taiwan in 2010, carry ARGs in dif modules. Only two plasmids from lineage 2 appear to have acquired additional ARGs: KT852971 has acquired a sul1-containing class 1 integron with cassette array aadB-arr-2-cmlA1-aadA1 and JX101647 has acquired sul2 and aphA1 in an insertion within an existing ARG-containing dif module, described below.
To assess whether and how individual dif modules have evolved since they were acquired by the ancestor of lineage 2 plasmids, their ARG-containing dif modules were compared. The msr(E)-mph(E) module was unchanged between the four plasmids that carried it, but variation was seen across bla OXA-58 (Fig. 3a), sul2 (Fig. 3b) and aacC2d-containing (Fig. 3c) modules. The novel IS elements ISAso1 and ISAso2, members of the uncharacterized ISNCY family, were acquired by both the bla OXA-58 and sul2-containing modules in JX101647 (Fig. 3). Both IS inserted in the same orientation adjacent to the XerC binding ends of pdif sites that flank their respective dif modules (Fig. 3a, b), with the 5 bp immediately adjacent to pdif involved in the target site duplication generated by insertion. The IS6/IS26-family element IS1008 fused the bla OXA-58-containing module of CP027245 to the remnant of a previously described RND efflux module [11] following a deletion of indeterminate length (Fig. 3a). IS1008 also deleted part of the sul2-containing module of CP028560 and brought the remainder adjacent to another sequence, possibly a remnant of a floR-containing module as IS1008 has truncated the floR gene (Fig. 3b). In JX101467 a partial deletion of the ISAba14-like element is associated with the acquisition of a 12213 bp segment bounded at one end by an ISOur1-like element and at the other by an ISAlw27-like element (Fig. 3c). The acquired segment includes sul2 and a truncated copy of Tn5393 that is interrupted by the aphA1-containing Tn4352. These examples highlight the capacity of IS to influence the accessory content of dif modules through insertion and by mediating deletion events.
Fig. 3.
ARG-containing dif module variants. Scaled diagrams of dif modules containing (a) bla OXA-58, (b) sul2 and (c) aacC2d. The extents and orientations of ORFs are indicated by labelled horizontal arrows and IS are shown as labelled boxes. IS that are the same colour belong to the same family. Drawn to scale from GenBank accessions CP072528, JX101647, CP028560, KT852971 and CP027245.
Diverse accessory genes are found in dif modules
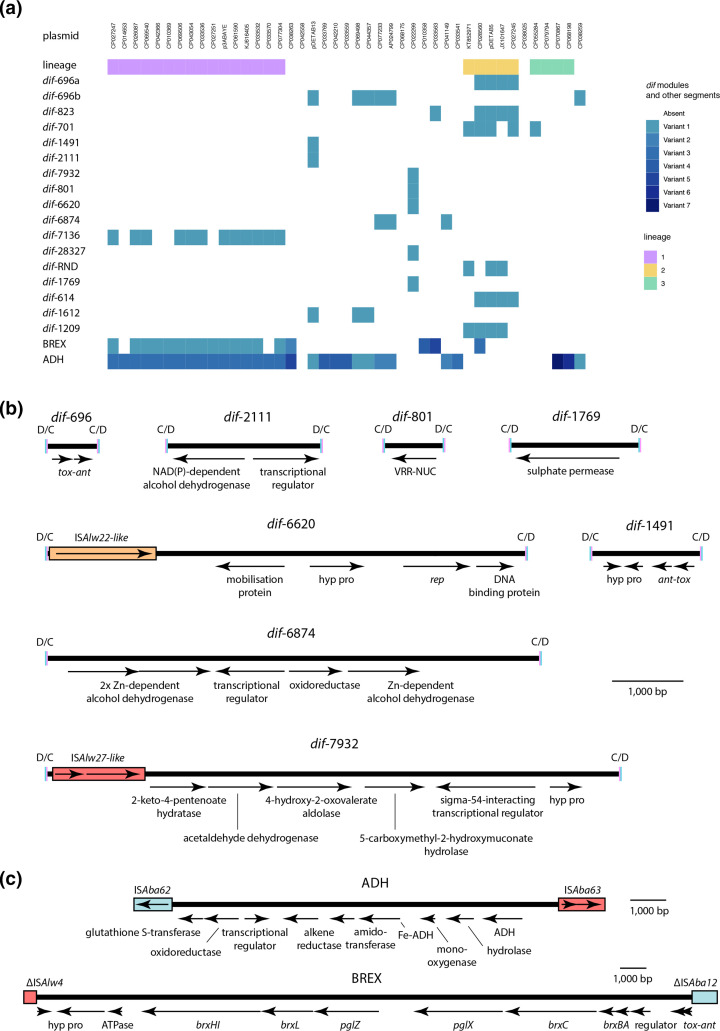
To further characterize their potential for mobilizing accessory genes other than the well-known ARGs, we examined the content and distribution of 17 dif modules identified in pDETAB5, pDETAB13, p3ABAYE, AP024799, CP022299 and CP068175 (Table S6). The sequences of these modules were used to query the GR13 collection with blastn and their distributions are shown in Fig. 4(a). Twelve dif modules were only carried by the plasmid or plasmid lineage that they were identified in, but five were found in multiple lineages, suggesting that they have been acquired independently.
Fig. 4.
Novel dif modules carrying diverse accessory genes. (a) Presence and absence of dif modules identified in GR13 plasmids. Plasmids are ordered as in the rep gene phylogeny shown in Fig. 2, and membership of lineages 1, 2 and 3 is indicated. The presence of variant BREX and ADH segments are indicated by different shades of colour (b) Scaled diagrams of selected dif modules. (c) Scaled diagrams of accessory gene segments. Sequences in parts (b) and (c) were drawn to scale from GenBank accessions CP072528, CP073061, CU459140, AP024799, CP022299 and CP068175.
Three dif modules identified here (dif-2111, dif-6874 and dif-7136) encode putative alcohol dehydrogenases, which may be involved with alcohol tolerance and quorum sensing [42]. The largest module we identified, dif-28327, encodes putative copper resistance proteins, and dif-7932 encodes a set of putative metabolic proteins that appear to be involved with aromatic compound degradation (Fig. 4b). The dif-1769 module carries a putative sulphate permease determinant and is 88 % identical to part of a sulP module that has been described previously [26]. The dif-6620 module found only in the cointegrate plasmid CP022299 contains a rep gene 82.3 % identical to the reference GR26 rep (GenBank accession CP015365), as well as a putative mobilisation gene (Fig. 4b). This appears to be a small plasmid that has been integrated through recombination at pdif sites. The remaining modules could not be assigned putative functions, though one of these, dif-801, encodes a protein with a VRR-NUC domain (Pfam: PF08774), equivalents to which have been described in restriction endonucleases (43).
Two sets of ORFs from the GR13 shell genome that appeared to be discrete units in the genome network were examined to determine whether they were found in well-conserved dif modules. The first set of ORFs resemble determinants for bacteriophage exclusion (BREX) systems [44], and likely have anti-phage functions. The BREX determinants are not part of an identifiable dif module. Instead, they are found in a 26 140 bp segment flanked by partial copies of ISAlw4 and ISAba12 (Fig. 4c). The same BREX segment is present in 14 of 16 plasmids from lineage 1, while variant sequences are present in four plasmids from elsewhere in the phylogeny (Fig. 4a). The second set of well-conserved ORFs correspond to the ADH segment of pDETAB13 that contains two putative alcohol dehydrogenase determinants, as well as several ORFs with expected metabolic functions and one for a putative transcriptional regulator (Fig. 4c). Variants of the ADH segment are found in 30 of the 43 GR13 plasmids studied here (Fig. 4a).
Diverse insertion sequences shape GR13 plasmid accessory content
We used a version of the ISFinder database to screen the GR13 plasmid collection and assess the abundance, diversity and richness of IS. Individual plasmids contained between one and 34 IS, with up to 19 different IS and up to six copies of the same IS found in a single plasmid (Fig. S4). Sixty-four different IS were found, representing 15 different IS families. These included 17 novel IS that differed from named sequences by greater than 5 % nucleotide identity and were characterized, submitted to ISFinder and assigned names as part of this study (Fig. S4, Table S2).
Members of the IS3 (17 different IS) and IS5 (12 different IS) families dominated the IS population, with representatives of one or both found in all but two GR13 plasmids. The next best-represented family was IS6/IS26 (6 different IS), members of which are known to be strongly associated with antibiotic resistance genes [45]. The highest numbers of IS6/IS26 family elements were found in the ARG-containing lineage 2 plasmids, where many were associated with ARG-containing dif modules (green in Fig. 3), but these elements were also seen in 13 plasmids that do not contain ARGs. ISNCY-family IS (7 different IS; 26 copies) including ISAlw22, ISAso1, ISAso2 (Figs. 3 and 4B), and 4 more novel IS identified here are distributed throughout the GR13 family.
Discussion
Our discovery of two GR13-type plasmids in unrelated A. baumannii strains isolated a month apart in the same ICU, one cryptic and the other conferring multi-drug resistance, prompted an investigation of the wider GR13 plasmid family, which had not been studied previously. GR13 plasmids are found in multiple Acinetobacter species from a diverse set of environments. The four-gene core of GR13 plasmids is associated with a diverse accessory genome influenced by the exchange of dif modules, the acquisition of translocatable elements and IS-mediated deletions. This characterization of a family of Acinetobacter plasmids that can carry clinically significant ARGs adds to a growing body of literature on the accessory genepool of this important human pathogen and the role of plasmids in generating diversity across this genus.
Diversity in GR13 plasmids: consequences for genomic surveillance and epidemiology
We identified three GR13 lineages on the basis of rep gene identity that we found share lineage-specific sets of accessory genes. Although rep or core-gene typing cannot account for all accessory gene diversity within GR13 lineages, we found that plasmids in the same lineage share significant gene content. Lineage-specific markers like rep and parAB might be used in targeted surveillance programmes to detect clinically relevant plasmids such as pDETAB5 and other members of lineage 2. Representatives of lineage 2 have so far only been seen in isolates from China or neighbouring Vietnam (Table S5), where the first example appeared in 2005, but it will be interesting to trace this lineage and monitor the dynamics of its dispersal in epidemiological studies. We have provided the sequences of the rep and parAB genes that can be used to identify plasmids from lineages 1, 2 and 3 in File S4. These can be used to track the dissemination of GR13 lineages across Acinetobacter .
How do GR13 plasmids spread horizontally?
Plasmids from the same GR13 lineages have been found in different host species that have been isolated from various sources and geographic locations. This is clear evidence for their widespread dissemination and ability to replicate in various Acinetobacter species. However, the mechanisms responsible for the horizontal transmission of GR13 plasmids remain unclear. In this study, pDETAB5 transferred from DETAB-E227 to A. baumannii ATCC 17978 at a relatively low frequency, but failed to transfer from DETAB-E227 or ATCC 17978 to A. nosocomialis strain XH1816.
No candidate set of ORFs for a type IV secretion system that might be associated with conjugation were found in pDETAB5 or any of the GR13 plasmids examined here, so it appears they rely on alternative mechanisms for horizontal transfer. In contrast, other large Acinetobacter plasmids have been shown to carry conjugation determinants in conserved backbones [13, 16, 46] while small plasmids that carry an origin-of-transfer (oriT) and cognate mobilisation genes [25] or oriT alone [47] can be mobilized by co-resident conjugative plasmids. It is possible that the integration of small mobilisable plasmids through recombination at pdif sites contributes to the mobility of GR13 plasmids. An example of this is seen in CP022299 where all or part of a putatively mobilisable plasmid is present in the dif-6620 module (Fig. 4). The acquisition of oriT sequences through small plasmid integration has been described for large plasmids in Staphylococcus and Proteus [48, 49], though in those cases integration did not involve pdif sites. Horizontal transfer in outer membrane vesicles has also been reported in Acinetobacter [50, 51] and this, or other passive DNA transfer mechanisms, might play a role in plasmid dispersal. The potential role of ATCC 17978’s resident plasmids in horizontal transfer of pDETAB5 was not examined experimentally here, so we cannot exclude the possibility that these played a role.
Site-specific recombination and tyrosine recombinase genes in GR13 plasmids
The importance of site-specific recombination to the evolution of some types of plasmids in Acinetobacter has become increasingly evident. XerC and XerD-mediated recombination at pdif sites is implicated in the movement of dif modules between plasmids of different types [12, 25, 52], and has been shown experimentally to generate cointegrate plasmids [53]. Recombination at pdif sites can also resolve cointegrates, potentially generating hybrids of the initial cointegrate-forming molecules [53]. This process likely explains the striking similarity of the GR13 plasmid pDETAB5 and GR34 plasmid pDETAB2 (Fig. 1b). Supporting this hypothesis, another plasmid examined here, CP028560, is a cointegrate with GR13 and GR34 replicons identical to those in pDETAB5 and pDETAB2, and appears to represent an evolutionary intermediate.
Given pdif sites appear to play a major role in the evolution of some plasmids, it will be important to define the types of plasmids that carry them and can participate in XerC/D-mediated cointegration events or the exchange of dif modules. A recently characterized family of Acinetobacter plasmids has pangenome characteristics similar to the GR13 family, and representatives carry mosaic regions that were called ‘hotspots’ [54]. The movement of dif modules might explain the dynamics of these hotspot regions. It will be useful to identify and study specific pdif-containing plasmid lineages over sustained periods of time to track small-scale evolutionary changes and further our understanding of the evolutionary consequences of pdif carriage.
The dif module gene repertoire continues to grow
The first-described dif modules contained ARGs, but further studies have revealed that these mobile elements can carry a diverse array of passenger genes. Our characterization of selected dif modules in GR13 plasmids expands the known repertoire of genes associated with these elements, further highlighting their important role in the diversification of the Acinetobacter accessory genome.
Many modules with diverse functions, including those expected to contribute to clinically relevant traits such as antibiotic resistance or alcohol tolerance, are accompanied by one or more dif modules carrying toxin–antitoxin genes [11, 12, 25]. These are expected to contribute to the stability of their host plasmids, and therefore to co-resident dif modules. ORFs with toxin–antitoxin functions made up 16 % of functionally annotated gene families in GR13 plasmids, suggesting that they play an important role in plasmid persistence. Diversity seen in toxin–antitoxin modules here and elsewhere support the hypothesis that these and other dif modules are ancient elements that have co-evolved with the plasmids of Acinetobacter [25].
Insertion sequences target and reshape dif module-containing plasmids
By definition IS do not encode proteins other than those required for their transposition, but their actions can profoundly influence the evolution of their host molecules [55]. In this study we found cases where IS that are expected to generate target site duplications on insertion are not flanked by them, suggesting that they have mediated deletion events. These deletion events have clearly been responsible for sequence loss from dif modules, or the fusion of dif modules to other sequences (Fig. 3). It appears IS-mediated deletion events can produce novel, hybrid dif modules, though whether these are mobile is likely to depend on the precise sequences of their new flanking pdif sites [52]. IS-mediated deletions might also remove pdif sites associated with dif modules, creating larger segments that might resemble the IS-flanked BREX and ADH segments (Fig. 4c).
Two elements characterized here, ISAso1 and ISAso2, are distantly related to one another (encoding 71.0 % identical transposases), but inserted in the same orientation immediately adjacent to the XerC binding regions of pdif sites (Fig. 3). A further four elements characterized here (ISAba69, ISAba70, ISAjo5 and ISApi2) are related to ISAso1 and ISAso2 and appear to exhibit the same target specificity. Together with previous descriptions of ISAjo2-like elements [12, 25], our findings support the notion that this group of IS are ‘dif site hunters’. The presence of dif site hunters can be considered strongly indicative of the presence of pdif sites in Acinetobacter plasmids, and might aid in the identification of plasmid types that participate in the exchange of dif modules.
Conclusions
GR13 plasmids have the capacity to accumulate diverse accessory sequences that may provide fitness advantages in the array of environments inhabited by Acinetobacter species. Some accessory modules pose risks to human health as they might contribute to the persistence of Acinetobacter populations in hospital environments. GR13 plasmid lineages have disseminated internationally and amongst different Acinetobacter species. Genomic surveillance should be coupled with experimental characterization of these plasmids to better understand their contribution to the diversification and success of Acinetobacter , particularly in nosocomial settings.
Supplementary Data
Funding information
This work was undertaken as part of the DETECTIVE research project funded by the National Natural Science Foundation of China (81861138054, 82072313, 31970128), Zhejiang Province Medical Platform Backbone Talent Plan (2020RC075) and the Medical Research Council (MR/S013660/1). W.v.S was also supported by a Wolfson Research Merit Award (WM160092).
Acknowledgements
We are grateful to the doctors and nurses in the ICU for sample collection. We thank Professor Zhiyong Zong and his team for their careful teaching of sampling methods.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: GR, rep group; IS, insertion sequence; pdif, plasmid-dif; SNP, single nucleotide polymorphism.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Four supplementary figures and six supplementary tables are available with the online version of this article.
References
- 1.Moran RA, Liu H, Doughty EL, Hua X, Cummins EA, et al. GR13-type plasmids in Acinetobacter potentiate the accumulation and horizontal transfer of diverse accessory genes. Figshare. 2022 doi: 10.6084/m9.figshare.19494596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visca P, Seifert H, Towner KJ. Acinetobacter infection--an emerging threat to human health. IUBMB Life. 2011;63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 3.Hamidian M, Nigro SJ. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii . Microb Genom. 2019;5 doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Endo S, Yano H, Kanamori H, Inomata S, Aoyagi T, et al. High frequency of Acinetobacter soli among Acinetobacter isolates causing bacteremia at a tertiary hospital in Japan. J Clin Microbiol. 2014;52:911–915. doi: 10.1128/JCM.03009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal S, Sharma M, Yadav A, Bala K, Chaudhary U. Acinetobacter lwoffii an emerging pathogen in neonatal ICU. Infect Disord Drug Targets. 2015;15:184–188. doi: 10.2174/1871526515666150826114745. [DOI] [PubMed] [Google Scholar]
- 6.Sieswerda E, Schade RP, Bosch T, de Vries J, Chamuleau MED, et al. Emergence of carbapenemase-producing Acinetobacter ursingii in The Netherlands. Clin Microbiol Infect. 2017;23:779–781. doi: 10.1016/j.cmi.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Silva L, Mourão J, Grosso F, Peixe L. Uncommon carbapenemase-encoding plasmids in the clinically emergent Acinetobacter pittii . J Antimicrob Chemother. 2018;73:52–56. doi: 10.1093/jac/dkx364. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Dong N, Xu C, Ye L, Chen S. Emergence of ST63 pandrug-resistant Acinetobacter pittii isolated from an AECOPD patient in China. Front Cell Infect Microbiol. 2021;11:739211. doi: 10.3389/fcimb.2021.739211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertini A, Poirel L, Mugnier PD, Villa L, Nordmann P, et al. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii . Antimicrob Agents Chemother. 2010;54:4168–4177. doi: 10.1128/AAC.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado-Camargo AD, Castro-Jaimes S, Gutierrez-Rios R-M, Lozano LF, Altamirano-Pacheco L, et al. Structure and evolution of Acinetobacter baumannii plasmids. Front Microbiol. 2020;11:1283. doi: 10.3389/fmicb.2020.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Moran RA, Chen Y, Doughty EL, Hua X, et al. Transferable Acinetobacter baumannii plasmid pDETAB2 encodes OXA-58 and NDM-1 and represents a new class of antibiotic resistance plasmids. J Antimicrob Chemother. 2021;76:1130–1134. doi: 10.1093/jac/dkab005. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell GA, Hall RM. The tet39 determinant and the msrE-mphE genes in Acinetobacter plasmids are each part of discrete modules flanked by inversely oriented pdif (XerC-XerD) sites. Antimicrob Agents Chemother. 2017;61:e00780–17. doi: 10.1128/AAC.00780-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamidian M, Ambrose SJ, Hall RM. A large conjugative Acinetobacter baumannii plasmid carrying the sul2 sulphonamide and strAB streptomycin resistance genes. Plasmid. 2016;87–88:43–50. doi: 10.1016/j.plasmid.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Hamidian M, Hall RM. pACICU2 is a conjugative plasmid of Acinetobacter carrying the aminoglycoside resistance transposon TnaphA6 . J Antimicrob Chemother. 2014;69:1146–1148. doi: 10.1093/jac/dkt488. [DOI] [PubMed] [Google Scholar]
- 15.Liu L-L, Ji S-J, Ruan Z, Fu Y, Fu Y-Q, et al. Dissemination of bla OXA-23 in Acinetobacter spp. in China: main roles of conjugative plasmid pAZJ221 and transposon Tn2009 . Antimicrob Agents Chemother. 2015;59:1998–2005. doi: 10.1128/AAC.04574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro SJ, Hall RM. Amikacin resistance plasmids in extensively antibiotic-resistant GC2 Acinetobacter baumannii from two Australian hospitals. J Antimicrob Chemother. 2014;69:3435–3437. doi: 10.1093/jac/dku310. [DOI] [PubMed] [Google Scholar]
- 17.Alattraqchi AG, Mohd Rani F, A Rahman NI, Ismail S, Cleary DW, et al. Complete genome sequencing of Acinetobacter baumannii AC1633 and Acinetobacter nosocomialis AC1530 unveils a large multidrug-resistant plasmid encoding the NDM-1 and OXA-58 carbapenemases. mSphere. 2021;6:e01076-20. doi: 10.1128/mSphere.01076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi W, Iimura M, Horiuchi K, Arai E, Natori T, et al. Occurrence of bla NDM-1 in a clinical isolate of Acinetobacter lwoffii in Japan: comparison of bla NDM-1-harboring plasmids between A. lwoffii and A. pittii Originated from A Hospital Sink. Jpn J Infect Dis. 2021;74:252–254. doi: 10.7883/yoken.JJID.2020.806. [DOI] [PubMed] [Google Scholar]
- 19.Li L-H, Yang Y-S, Sun J-R, Huang T-W, Huang W-C, et al. Clinical and molecular characterization of Acinetobacter seifertii in Taiwan. J Antimicrob Chemother. 2021;76:312–321. doi: 10.1093/jac/dkaa432. [DOI] [PubMed] [Google Scholar]
- 20.Balalovski P, Grainge I. Mobilization of pdif modules in Acinetobacter: A novel mechanism for antibiotic resistance gene shuffling? Mol Microbiol. 2020;114:699–709. doi: 10.1111/mmi.14563. [DOI] [PubMed] [Google Scholar]
- 21.Lin DL, Traglia GM, Baker R, Sherratt DJ, Ramirez MS, et al. Functional analysis of the Acinetobacter baumannii XerC and XerD site-specific recombinases: potential role in dissemination of resistance genes. Antibiotics (Basel) 2020;9:405. doi: 10.3390/antibiotics9070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Andrea MM, Giani T, D’Arezzo S, Capone A, Petrosillo N, et al. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii . Antimicrob Agents Chemother. 2009;53:3528–3533. doi: 10.1128/AAC.00178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertini A, Poirel L, Bernabeu S, Fortini D, Villa L, et al. Multicopy bla OXA-58 gene as a source of high-level resistance to carbapenems in Acinetobacter baumannii . Antimicrob Agents Chemother. 2007;51:2324–2328. doi: 10.1128/AAC.01502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo H-Y, Hsu P-J, Chen J-Y, Liao P-C, Lu C-W, et al. Clonal spread of bla OXA-72-carrying Acinetobacter baumannii sequence type 512 in Taiwan. Int J Antimicrob Agents. 2016;48:111–113. doi: 10.1016/j.ijantimicag.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Hamidian M, Hall RM. Genetic structure of four plasmids found in Acinetobacter baumannii isolate D36 belonging to lineage 2 of global clone 1. PLoS One. 2018;13:e0204357. doi: 10.1371/journal.pone.0204357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mindlin S, Petrenko A, Petrova M. Chromium resistance genetic element flanked by XerC/XerD recombination sites and its distribution in environmental and clinical Acinetobacter strains. FEMS Microbiol Lett. 2018;365 doi: 10.1093/femsle/fny047. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Wang R, Wang X, Wang Q, Zhang Y, et al. Emergence of mcr-1 and carbapenemase genes in hospital sewage water in Beijing, China. J Antimicrob Chemother. 2018;73:84–87. doi: 10.1093/jac/dkx355. [DOI] [PubMed] [Google Scholar]
- 28.Quan J, Li X, Chen Y, Jiang Y, Zhou Z, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017;17:400–410. doi: 10.1016/S1473-3099(16)30528-X. [DOI] [PubMed] [Google Scholar]
- 29.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartual SG, Seifert H, Hippler C, Luzon MAD, Wisplinghoff H, et al. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii . J Clin Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–6. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 35.Tonkin-Hill G, MacAlasdair N, Ruis C, Weimann A, Horesh G, et al. Producing polished prokaryotic pangenomes with the Panaroo pipeline. Genome Biol. 2020;21:180. doi: 10.1186/s13059-020-02090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47:D309–D314. doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol. 2021;38:5825–5829. doi: 10.1093/molbev/msab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchfink B, Reuter K, Drost H-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods. 2021;18:366–368. doi: 10.1038/s41592-021-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sela I, Ashkenazy H, Katoh K, Pupko T. GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 2015;43:W7–14. doi: 10.1093/nar/gkv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin G-H, Hsieh M-C, Shu H-Y. Role of iron-containing alcohol dehydrogenases in Acinetobacter baumannii ATCC 19606 stress resistance and virulence. Int J Mol Sci. 2021;22:9921. doi: 10.3390/ijms22189921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinch LN, Ginalski K, Rychlewski L, Grishin NV. Identification of novel restriction endonuclease-like fold families among hypothetical proteins. Nucleic Acids Res. 2005;33:3598–3605. doi: 10.1093/nar/gki676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldfarb T, Sberro H, Weinstock E, Cohen O, Doron S, et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015;34:169–183. doi: 10.15252/embj.201489455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmer CJ, Hall RM. An analysis of the IS6/IS26 family of insertion sequences: is it a single family? Microb Genom. 2019;5 doi: 10.1099/mgen.0.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nigro SJ, Holt KE, Pickard D, Hall RM. Carbapenem and amikacin resistance on a large conjugative Acinetobacter baumannii plasmid. J Antimicrob Chemother. 2015;70:1259–1261. doi: 10.1093/jac/dku486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blackwell GA, Hall RM. Mobilisation of a small Acinetobacter plasmid carrying an oriT transfer origin by conjugative RepAci6 plasmids. Plasmid. 2019;103:36–44. doi: 10.1016/j.plasmid.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Hua X, Zhang L, Moran RA, Xu Q, Sun L, et al. Cointegration as a mechanism for the evolution of a KPC-producing multidrug resistance plasmid in Proteus mirabilis . Emerg Microbes Infect. 2020;9:1206–1218. doi: 10.1080/22221751.2020.1773322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien FG, Yui Eto K, Murphy RJT, Fairhurst HM, Coombs GW, et al. Origin-of-transfer sequences facilitate mobilisation of non-conjugative antimicrobial-resistance plasmids in Staphylococcus aureus . Nucleic Acids Res. 2015;43:7971–7983. doi: 10.1093/nar/gkv755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the bla NDM-1 gene via outer membrane vesicles. J Antimicrob Chemother. 2017;72:2201–2207. doi: 10.1093/jac/dkx131. [DOI] [PubMed] [Google Scholar]
- 51.Rumbo C, Fernández-Moreira E, Merino M, Poza M, Mendez JA, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii . Antimicrob Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamidian M, Ambrose SJ, Blackwell GA, Nigro SJ, Hall RM. An outbreak of multiply antibiotic-resistant ST49:ST128:KL11:OCL8 Acinetobacter baumannii isolates at a Sydney hospital. J Antimicrob Chemother. 2021;76:893–900. doi: 10.1093/jac/dkaa553. [DOI] [PubMed] [Google Scholar]
- 53.Cameranesi MM, Morán-Barrio J, Limansky AS, Repizo GD, Viale AM. Site-specific recombination at XerC/D sites mediates the formation and resolution of plasmid co-integrates carrying a bla OXA-58- and TnaphA6-resistance module in Acinetobacter baumannii . Front Microbiol. 2018;9:66. doi: 10.3389/fmicb.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghaly TM, Paulsen IT, Sajjad A, Tetu SG, Gillings MR. A novel family of Acinetobacter mega-plasmids are disseminating multi-drug resistance across the globe while acquiring location-specific accessory genes. Front Microbiol. 2020;11:605952. doi: 10.3389/fmicb.2020.605952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vandecraen J, Chandler M, Aertsen A, Van Houdt R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit Rev Microbiol. 2017;43:709–730. doi: 10.1080/1040841X.2017.1303661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.