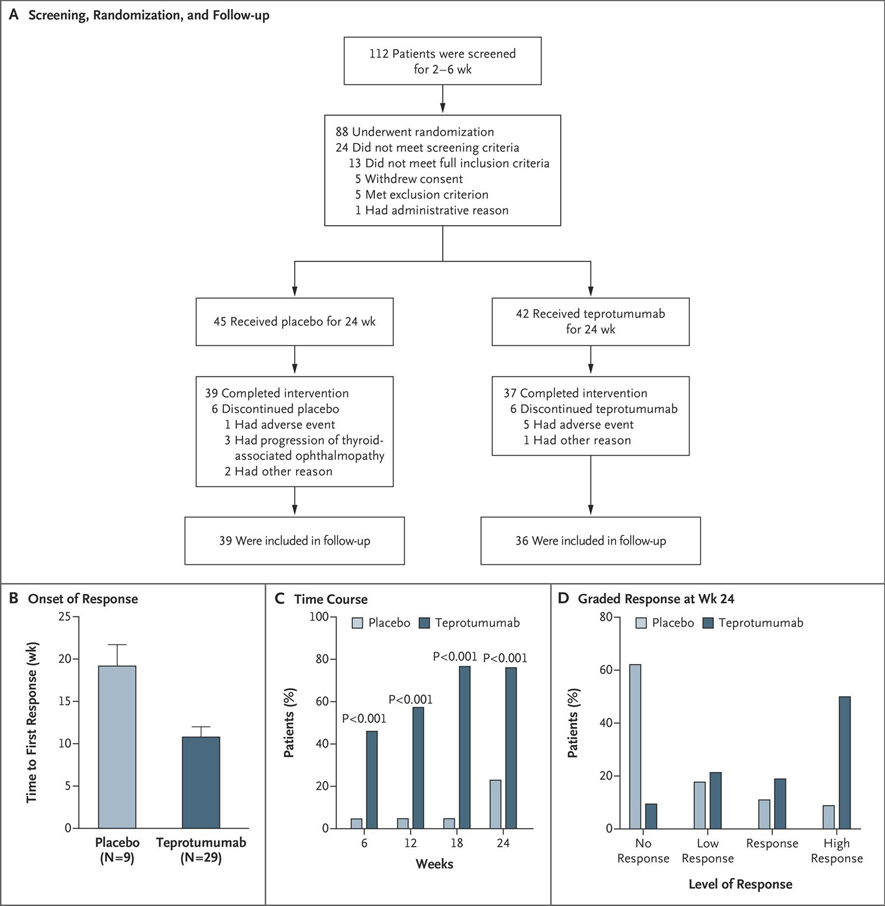
Figure 2.
Phase II trial design and therapeutic response. (a) Strategy for screening patients, their randomization into the two treatment arms for a 24-week intervention phase, and follow-up. One patient after screening failed to meet inclusion criteria, and withdrew prior to treatment initiation. (b) Analysis of the primary response (a reduction of ≥ 2 mm in proptosis and an improvement of ≥ 2 points on a 7-point scale in clinical activity score) in the study eye. (c) Time course in patients meeting response criteria at 6,12,18 and 24 weeks during treatment. (d) Grading the response at week 24. A high response denoted decrease of proptosis ≤ 3 mm and clinical activity score reduction ≤ 3 points. A response indicates a reduction ≥ 2 mm but < 3 mm in proptosis, and ≥ 2 points but < 3 points in Clinical Activity Score. A low response indicates reductions ≥ 1 mm but < 2 mm in proptosis and ≥ 1 point but < 2 points in Clinical Activity Score. No response indicates that the patient did not meet any response criteria or missed evaluation at week 24. Reprinted with permission from Smith TJ et al N Engl J Med. 2017; copyright 2017 Massachusetts Medical Society